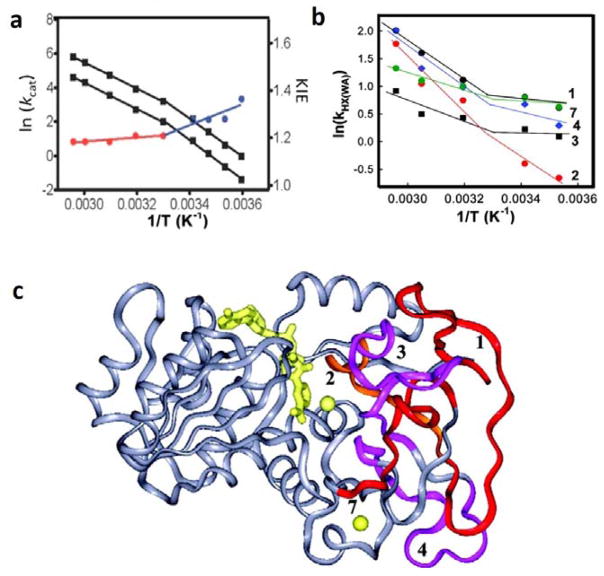
Figure 9.
Functionally relevant temperature dependent breaks in behavior for ht-ADH. In (a), illustration of the kinetic break at 30 ˚C for both the kcat and the KIE (i.e. Dkcat). The KIE is almost temperature independent above 30 ˚C (red) and transitions to a more temperature dependent KIE behavior below 30 ˚C (blue) with a concomitant increase in Ea for kcat.69 In (b), HDXMS analysis revealed 5 peptides (1–4, 7) that show an analogous temperature dependent transition in k[HX(WA)].71 In (c), these five peptides are numbered, mapped onto the ht-ADH structure and colored as red, orange, and magenta. The active-site Zn2+ is shown as a yellow sphere, adjacent to peptide 2, and the cofactor NAD+ is represented as yellow sticks.