Abstract
Objective
The purpose of this study was to investigate the difference between arterialized and venous blood lactate concentrations [La] during constant-load exercises at different intensities.
Methods
Fifteen physically active men cycled for 30 minutes (or until exhaustion) at the first lactate threshold (LT1), at 50% of the difference between the first and second lactate threshold (TT50%), at the second lactate threshold (LT2), and at 25% of the difference between LT2 and maximal aerobic power output (TW25%). Samples of both arterialized and venous blood were collected simultaneously at rest and every 5 minutes during the exercise.
Results
The arterialized blood [La] was higher at minute 5 than venous blood [La] for all exercise intensities (p < 0.05). After this period, the arterialized and venous [La] samples became similar until the end of the exercise (p > 0.05). The arterialized-venous difference during the first 10 minutes was greater for the two highest exercise intensities (LT2 and TW25%) compared with the two lowest (LT1 and TT50%, p < 0.05). Thereafter, arterialized-venous difference decreased progressively, reaching values close to zero for all exercise intensities (p > 0.05).
Conclusion
These results suggest a delayed lactate appearance in the venous blood, which is accentuated at higher exercise intensities. The lactate measured in arterialized and venous blood is interchangeable only when blood samples are collected at least 10 minutes after the exercise starts.
Keywords: Anaerobic threshold, Arterialized-venous difference, Exercise domains, Lactate balance, Lactate clearance, Physiological assessment
1. Introduction
Blood lactate concentration ([La]) is a measure widely used to determine training intensity zones, quantify the effects of endurance training and/or to estimate the lactic energy expenditure of a given activity task.1, 2, 3 This blood [La] is the result of the balance between its appearance and removal.4, 5 Lactate can be produced by red blood cells, by the brain, by the gut, and by the skin. During exercise, however, the main tissues producing lactate are the active muscles. Lactate produced during exercise is continually cleaned up by the inactive muscles, heart, liver and kidney.4, 5, 6
The gold-standard approach to measure lactate balance (release and uptake) is by the exercising lower limb muscles (e.g., cycling). This is done by taking femoral arterial and femoral venous blood samples while measuring femoral arterial blood flow. However, the access to the femoral artery and femoral vein is highly invasive, uncomfortable and technically difficult during whole-body exercise.7, 8 An alternative is to collect samples from the brachial artery, where the La matches the values observed in the femoral vein.9 The most visible access sites are the small-caliber arm arteries, although they are easily missed during exercise, and demand a procedure which causes great discomfort.10 Because of the cumbersome nature of these blood-collecting sites, the collection of femoral arterial/venous and brachial artery blood samples is not routine during either physiological research or diagnostic exercise testing. Rather, the most widely used collection sites are arterialized blood samples from easier-access sites such as the earlobe or fingertip, or venous blood samples from the arm veins. Arterialized blood samples may be collected in micro samples from these sites, with minimal discomfort.11, 12 During incremental exercise, measurements of the [La] taken from the arterialized capillary blood samples seems to be similar to both the arterial blood [La] collected from the brachial artery and venous blood collected from antecubital vein.9 On the other hand, it has also been observed that the blood [La] concentration in the arm vein is slightly lower than the blood [La] in arterialized capillary during the highest stages of an incremental exercise.13 Similarly, concentrations in venous blood samples collected from the forearm vein during an incremental exercise seem also to be slightly lower than in the arterial blood [La] collected from the forearm artery, and the arterio-venous lactate difference becomes greater with the increase in the exercise intensity.8
Therefore, an important aspect that may contribute to maximize differences between arterialized capillary and arm venous blood [La] is the intensity of the exercise; this, however, has not been widely explored. An increase in intensity of the exercise should simultaneously increase lactate production by the active muscles and decrease peripheral blood flow to inactive arm muscles.14, 15 A decrease in this peripheral blood flow might delay the appearance of lactate in the arm venous blood, which might accentuate the differences between [La] there and in the arterialized capillary.6
A delayed arm venous [La] response may result in errors when determining lactate thresholds or when estimating lactic energy expenditure during a given task.1, 3 Although arm vein and earlobe blood collections are widely used for [La] determination during exercise,16 these samples could have been interchangeably used and the venous-arterialized blood [La] relationship could have been influenced by the exercise intensity. The possible interrelationship between exercise intensity and exercise duration is also not known. The recent increase of interest in measuring energy system contributions in a variety of sports, in particular anaerobic lactic and alactic contributions, makes this an important area for study.17, 18, 19 Usually, the lactic portion of the anaerobic energy system is largely estimated from blood [La].20 Since anaerobic lactic contribution is both intensity and duration dependent, it is important to be aware of the blood collection sites and how they influence the blood [La] as well as influence the estimate of lactic energy expenditure during exercise of various intensities.
Therefore, the aim of this study was to determine the difference of blood [La] response in the arterialized earlobe capillary and antecubital vein during constant-load exercise performed at different intensities. We hypothesized that the venous blood [La] presents a delayed response compared to the arterialized capillary blood [La], and that this difference might increase as the exercise intensity increases.
2. Methods
2.1. Participants
Fifteen male participants (mean ± SD: 28.1 ± 4.5 years; 82.0 ± 9.7 kg; 177.7 ± 4.6 cm; 14.1 ± 5.8 % of body fat; and 41.8 ± 4.0 mL·kg−1·min−1 of VO2max) were recruited to participate in this study. The participants were all recreationally active and were familiar with the experimental procedures. The participants were accustomed to cycle on a cyclo ergometer but were not cyclists. Participants were firstly informed of the requirements, benefits and risks of the study and thereafter signed a consent form. The study was approved by the Ethics Committee for Human Studies of the School of Physical Education and Sport of the University of São Paulo.
2.2. Procedures
Each participant visited the laboratory on five different occasions, with 72-h interval between visits. In the first visit, height, body mass, and skinfolds were measured (chest, abdomen and thigh). Body density was estimated according to Jackson and Pollock's equation21 and converted to body fat percentage using Siri's equation.22 Then, the participants performed a maximal incremental test on a cycle ergometer (Standart Lannoy Ergometer, Godart-Statham, Bilthoven, Holland) to determine their first (LT1) and second (LT2) lactate thresholds, and the peak power output (PPO). In visits 2 to 5, participants performed constant workload trials at: 1) LT1; 2) 50% of the difference between the LT1 and LT2 (TT50%); 3) LT2 or; 4) 25% of difference between the LT2 and the PPO (TW25%).
The trials were performed randomly, and at the same time of day to avoid any effect of the circadian cycle.12 Participants were instructed to consume their usual diet before the experimental sessions and to take all tests in a postprandial state (i.e., the last meal was taken 2h before the trial). They were recommended to avoid intense exercise, and to avoid consumption of food and drinks containing caffeine or alcohol in the 24 h preceding the trials.
2.3. Maximal incremental test
The maximal incremental test started with a 3-min, warm-up at 50 W, followed by increments of 20 W every 3 min until exhaustion. Participants were verbally encouraged to give their maximal effort during the test. At the end of each stage, 25 μL of arterialized blood sample from the vasodilated earlobe (Finalgon, Boehringer Ingelheim, Germany) was collected and immediately analyzed for [La], by using an automatic analyzer (YSI 1500 Sport, Yellow Springs Instruments, Yellow Springs, OH). The [La] was plotted as a function of the workload, and the LT1 and LT2 were identified by a 3-segment linear regression.23, 24, 25 The 3-segment linear regression fitting was calculated using an interactive process with two initially unknown intercepts calculated from every possible combination of intersection. The intercepts that best shared the curve in three linear segments were assumed when the highest R2 value and the lowest residual sum of squares were attained. The LT1 was therefore defined as the workload corresponding to an initial change in the rate of lactate accumulation in the blood, while LT2 was defined as the workload corresponding to the second change in the rate of lactate accumulation.
2.4. Constant workload trials
Constant workload trials were performed at intensities corresponding to LT1, TT50%, LT2 and TW25%, for 30 minutes or until exhaustion (if individuals were unable to tolerate the target duration). These exercise intensities corresponded to the upper boundary limit of moderate domain, mid-range of heavy domain, upper boundary limit of heavy domain, and first-quarter of very heavy domain, respectively.24 Participants were instructed to maintain a pedal cadence between 60 and 70 rpm throughout the test. Exhaustion was defined when participants could not maintain a 60 rpm pedal cadence.
Before each test, a catheter was inserted into the antecubital vein for venous blood collection. The arterialized capillary samples were collected from the vasodilated earlobe (Finalgon, Boehringer Ingelheim, Germany). The venous (10 ml) and arterialized (25 μL) blood samples were collected simultaneously before the trial (at rest) and every 5 min during the trials, with the last sample taken at minute 30 or exhaustion. The venous and arterialized blood [La] was immediately determined in the automatic analyzer (YSI 1500 Sport, Yellow Springs Instruments, Yellow Springs, OH).
2.5. Statistical analysis
The data are presented as means ± SE. Data distribution was analyzed by using the Shapiro-Wilk test and visual inspection of the Q-Q and box plots. Once normality was confirmed, two-way, repeated-measure ANOVA (having sampling site and time as fixed factors) was used to explore the differences between venous and arterialized blood [La] within exercise domains. Additionally, a two-way, repeated-measure ANOVA assessed the exercise intensity and duration effects for site-collection differences (delta difference between arterialized and venous [La]). If a main effect was observed, a Duncan post hoc test was applied to localize the difference. Statistical analysis was carried out using a Statistics package for Windows (StataSoft Inc®, version 10, Tulsa, OK, United States). The significance was set at p < 0.05.
3. Results
All participants were able to complete 30 min of exercise in LT1 and TT50%. In LT2 and TW25%, participants completed 27 ± 4.9 and 23 ± 7.2 min, respectively.
There was a main effect of time for all exercise intensities, with both arterialized and venous blood [La] increasing with the time (LT1: F6,144 = 54.45; TT50%: F6,162 = 46.14; LT2: F5,130 = 73.93 and TW25%: F3,84 = 109.2, all p < 0.05). Venous blood [La] in the LT1 increased gradually up to 10 min (p < 0.05) and then remained stable until the end of exercise (p > 0.05, Fig. 1A), whereas the arterialized blood [La] increased from rest to 5 min (p < 0.05), remained stable until minute 15 (p > 0.05), and then decreased slightly at minutes 20 and 25 (p < 0.05). In the TT50%, the venous blood [La] increased until minute 10 (p < 0.05) and then remained stable until the end of exercise (p > 0.05), whereas arterialized blood [La] increased until minute 5 (p < 0.05) and remained stable until the end of exercise (p > 0.05, Fig. 1B). Finally, the arterialized and venous blood [La] showed no stabilization and increased progressively until the end of the exercise in both LT2 and TW25% bouts (p < 0.05, Fig. 1C and D).
Fig. 1.
Venous (■) and arterialized (Օ) blood lactate concentration ([La]) response during exercise at LT1, TT50%, LT2, and TW25%. Data are presented as mean ± SE. Ɨ Venous blood [La] is significantly lower than arterialized blood [La] at the same time point (p < .05). * Significantly higher than at rest for both venous and arterialized blood [La] (p < .05). $ Lower than 10 and 5 min for arterialized blood [La] (p < .05). # Higher than 5 min for venous blood [La] (p < .05). LT1 = first lactate threshold; TT50% = 50% of the difference between LT1 and LT2; LT2 = second lactate threshold; TW25% = 25% of the difference between LT2 and peak power output.
There was an interaction between sampling site and time for all exercise intensities: LT1 (F6,144 = 4.01, p < 0.05); TT50% (F6,162 = 4.01, p < 0.05); LT2 (F5,130 = 3.21, p < 0.05) and TW25% (F3,84 = 4.12, p < 0.05). The arterialized blood [La] was higher than venous blood [La] only at minute 5 for all exercise intensities (p < 0.05, Fig. 1).
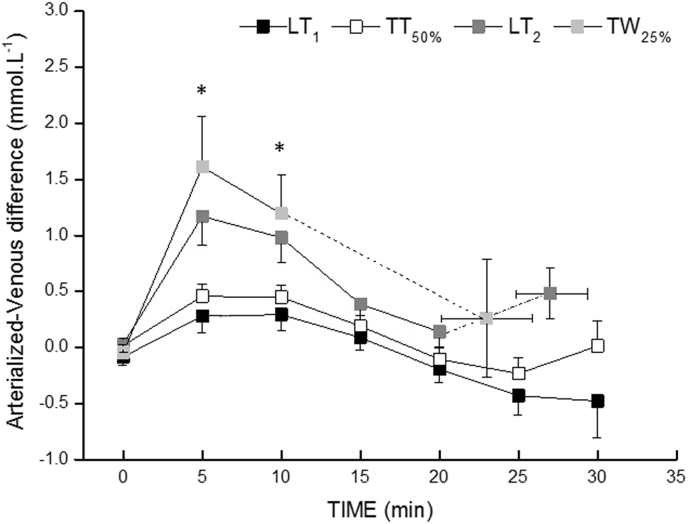
The arterialized-venous [La] difference at 5 and 10 minutes was higher in LT2 and TW25% compared to LT1 and TT50% (p < 0.05, Fig. 2), but there was no significant difference between LT1 and TT50% or between LT2 and TW25% (p > 0.05). The highest arterialized-venous difference in concentration was reached at 5 minutes, and then decreased progressively until the end of the exercises for all exercise intensities (p < 0.05).
Fig. 2.
Arterialized–venous difference for blood [La] during exercise at LT1, TT50%, LT2 and TW25%. * TW25% and LT2 are significantly higher than LT1 and TT50% at the same time point. LT1 = first lactate threshold; TT50% = 50% of the difference between LT1 and LT2; LT2 = second lactate threshold; TW25% = 25% of the difference between LT2 and peak power output.
4. Discussion
The purpose of this study was to investigate the response of the blood [La] collected in the antecubital vein and arterialized earlobe during constant-load exercise at different intensities. Our results demonstrate that blood [La] measured from the arterialized site is greater than that measured from the venous site only at the beginning of the exercise. The venous blood [La] matches the arterialized blood [La] thereafter. The arterialized–venous difference at the exercise commencement was more pronounced during the two highest exercise intensities (i.e., LT2 and TW25%), but it decreased progressively as the exercise progressed. This suggests that samples of venous and arterialized blood [La] cannot be used interchangeably, at least if the exercise is shorter than 10 minutes. This is of practical relevance in detecting discrepancies when comparing the results with those in the literature or when using absolute values for the calculation of lactic energy expenditure.
A number of studies have observed a significant difference between venous and arterialized blood [La].13, 26, 27 Using an incremental test protocol, Robergs et al.13 observed a delay (right displacement) of LT1 and LT2 when the blood [La] was measured from the venous as compared to the arterialized site. Foxdal et al.26 also observed that arterialized blood [La] was approximately 8% higher than venous blood [La] throughout an incremental exercise test. Similarly, Linderman et al.27 found higher blood [La] in arterialized sites compared to venous blood during exercise performed at VO2max intensity. However, the above-mentioned studies either performed an incremental exercise protocol or a single, high-intensity exercise bout, which precludes determining if the [La] differences between arterialized and venous blood occurred in an exercise-intensity and/or exercise-duration dependent manner. Our results show for the first time that the venous blood [La] takes at least ten minutes after the beginning of exercise to be matched with the arterialized blood [La], regardless of the exercise intensity.
The delayed venous blood [La] response at the beginning of exercise may have been associated with a vessel constriction in the less active tissues.6, 16 This would delay the flux of lactate to the peripheral veins. Although we did not measure forearm blood flow in the present study, previous studies have reported a drop in blood flow to peripheral regions during exercise, which in turn may cause a delay in the onset of lactate accumulation in the forearm vein.28, 29 In addition, even with a reduced blood flow to the inactive upper body muscles during cycling, arm muscles could increase lactate extraction from circulation, which in turn would contribute to an additional reduction of blood [La] in the antecubital or forearm veins.8, 30 Supporting this assumption, Jorfeldt30 observed that when lactate was infused into the brachial artery during light forearm exercise, approximately 30-50% of infused lactate was removed and oxidized by the arm and forearm muscles. Interestingly, in the present study, the arterialized-venous difference at the beginning of the exercise was between 30 to 43% across the different exercise intensities. This suggests that the inactive muscles of the arms are probably clearing and oxidizing some of the lactate produced in the legs thereby lowering the venous [La] relative to the arterialized sample. Thus, the clearance of lactate by inactive upper body muscles might have contributed to a significant decrease of blood [La] in the antecubital vein.8, 26, 30
The largest arterialized-venous differences were observed at LT2 and TW25%, confirming our hypothesis that arterialized-venous differences are likely to increase with the increase in the exercise intensity (Fig. 2). This would be predictable since an increased production of lactate by the intense muscle contraction creates a larger gradient between intra and extracellular space, thus diffusing lactate faster to the interstitial space.31, 32 In addition, an associated reduction in forearm blood flow and an increase in lactate extraction in the arm muscles would induce a larger arterialized-venous difference. The fact of venous blood [La] being lower than that of arterialized blood [La] is of practical relevance, if the interest is to quantify the contribution of the lactic energy system to total energy expenditure. For this, venous blood should not be taken before at least 10 minutes of exercise, mainly during high-intensity exercises. Otherwise, this will result in an underestimation of the lactic energy contribution. In addition, because incremental exercise with stages lasting no more than 2-3 minutes is usually applied in laboratory routines, a measurement of [La] from the venous blood will displace the lactate-workload curve to the right, which will carry over to an estimation of the lactate thresholds. Thus, a general recommendation provide by our results would be to use arterialized blood samples during the incremental exercise test or during constant-load exercise lasting less than 10 minutes. This is particularly important for high-intensity, constant-load exercises. However, the arterialized and venous blood [La] can be equally used when blood samples are collected after 10 minutes of exercise. However, the extrapolation of our results to intermittent exercise or sports practice should be made with caution, once the repeated periods of physiological stress followed by different periods of recovery might alter the pattern of appearance and removal of blood lactate.9 Therefore, the kinetics of lactate release and removal for intermittent exercises must be determined in further studies.
5. Conclusion
In conclusion, the venous blood [La] measured from the antecubital vein was less than that of blood [La] measured from the arterialized earlobe capillary only during the first five minutes of exercise. However, the arterialized-venous concentration difference was greater during the first 10 minutes of the two most intense exercises, suggesting that it takes at least 10 minutes of exercise to match lactate concentration in these two collecting sites. These results suggest that arterialized and venous [La] should be used interchangeably only when collected and measured 10 minutes after the exercise starts. Further studies should take in account these site-based differences when identifying lactate thresholds and/or quantifying the contribution of lactic energy during the exercise.
Conflict of interest
The authors declare no conflict of interest.
Funding/support
No financial or grant support was received for this work.
Acknowledgments
The authors would like to thank Dr. Rômulo Bertuzzi for his excellent technical assistance during data collection. The English text of this paper was revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University). All the authors declare that they have no conflict of interest derived from the outcomes of this study. No financial support was received.
References
- 1.di Prampero P.E., Ferretti G. The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respir Physiol. 1999;118:103–115. doi: 10.1016/s0034-5687(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson R.M., Sleivert G.G. Indices of lactate threshold and their relationship with 10-km running velocity. Med Sci Sports Exerc. 2001;33:339–342. doi: 10.1097/00005768-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Bertuzzi R.C., Franchini E., Ugrinowitsch C. Predicting MAOD using only a supramaximal exhaustive test. Int J Sports Med. 2010;31:477–481. doi: 10.1055/s-0030-1253375. [DOI] [PubMed] [Google Scholar]
- 4.Brooks G.A. The lactate shuttle during exercise and recovery. Med Sci Sports Exerc. 1986;18:360–368. doi: 10.1249/00005768-198606000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Granier P., Dubouchaud H., Mercier B. Lactate uptake by forearm skeletal muscles during repeated periods of short-term intense leg exercise in humans. Eur J Appl Physiol Occup Physiol. 1996;72:209–214. doi: 10.1007/BF00838640. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen H.B., Clemmesen J.O., Skak C. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. J Appl Physiol. 1985;2002(92):1677–1683. doi: 10.1152/japplphysiol.00028.2001. [DOI] [PubMed] [Google Scholar]
- 7.Bergman B.C., Wolfel E.E., Butterfield G.E. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T., Takeuchi N., Suda Y. Arterial versus venous blood lactate increase in the forearm during incremental bicycle exercise. Eur J Appl Physiol Occup Physiol. 1982;50:87–93. [Google Scholar]
- 9.Williams J.R., Armstrong N., Kirby B.J. The influence of the site of sampling and assay medium upon the measurement and interpretation of blood lactate responses to exercise. J Sports Sci. 1992;10:95–107. doi: 10.1080/02640419208729912. [DOI] [PubMed] [Google Scholar]
- 10.Dar K., Williams T., Aitken R. Arterial versus capillary sampling for analysing blood gas pressures. BMJ. 1995;310:24–25. doi: 10.1136/bmj.310.6971.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran P., Prichard J.G., Ansley L. The influence of blood lactate sample site on exercise prescription. J Strength Cond Res. 2012;26:563–567. doi: 10.1519/JSC.0b013e318225f395. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes A.L., Lopes-Silva J.P., Bertuzzi R. Effect of time of day on performance, hormonal and metabolic response during a 1000-M cycling time trial. PLoS One. 2014;9:e109954. doi: 10.1371/journal.pone.0109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robergs R.A., Chwalbinska-Moneta J., Mitchell J.B. Blood lactate threshold differences between arterialized and venous blood. Int J Sports Med. 1990;11:446–451. doi: 10.1055/s-2007-1024835. [DOI] [PubMed] [Google Scholar]
- 14.Gladden L.B. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas G.D., Segal S.S. Neural control of muscle blood flow during exercise. J Appl Physiol. 1985;2004(97):731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 16.Forster H.V., Dempsey J.A., Thomson J. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972;32:134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- 17.Campos F.A., Bertuzzi R., Dourado A.C. Energy demands in taekwondo athletes during combat simulation. Eur J Appl Physiol. 2012;112:1221–1228. doi: 10.1007/s00421-011-2071-4. [DOI] [PubMed] [Google Scholar]
- 18.Damasceno M.V., Pasqua L.A., Lima-Silva A.E. Energy system contribution in a maximal incremental test: correlations with pacing and overall performance in a 10-km running trial. Braz J Med Biol Res. 2015;48:1048–1054. doi: 10.1590/1414-431X20154787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beneke R., Leithauser R.M., Ochentel O. Blood lactate diagnostics in exercise testing and training. Int J Sports Physiol Perform. 2011;6:8–24. doi: 10.1123/ijspp.6.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Artioli G.G., Bertuzzi R.C., Roschel H. Determining the contribution of the energy systems during exercise. J Vis Exp. 2012 doi: 10.3791/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson A.S., Pollock M.L. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 22.Siri W.E. Body composition from fluid spaces and density: analysis of methods. Nutrition. 1961;1961(9):480–491. discussion 480, 492. [PubMed] [Google Scholar]
- 23.Ribeiro J.P., Hughes V., Fielding R.A. Metabolic and ventilatory responses to steady state exercise relative to lactate thresholds. Eur J Appl Physiol Occup Physiol. 1986;55:215–221. doi: 10.1007/BF00715008. [DOI] [PubMed] [Google Scholar]
- 24.Pires F.O., Noakes T.D., Lima-Silva A.E. Cardiopulmonary, blood metabolite and rating of perceived exertion responses to constant exercises performed at different intensities until exhaustion. Br J Sports Med. 2011;45:1119–1125. doi: 10.1136/bjsm.2010.079087. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Silva A.E., Bertuzzi R.C.M., Pires F.O. A low carbohydrate diet affects autonomic modulation during heavy but not moderate exercise. Eur J Appl Physiol. 2009;108:1133–1140. doi: 10.1007/s00421-009-1329-6. [DOI] [PubMed] [Google Scholar]
- 26.Foxdal P., Sjodin B., Rudstam H. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol Occup Physiol. 1990;61:218–222. doi: 10.1007/BF00357603. [DOI] [PubMed] [Google Scholar]
- 27.Linderman J., Fahey T.D., Lauten G. A comparison of blood gases and acid-base measurements in arterial, arterialized venous, and venous blood during short-term maximal exercise. Eur J Appl Physiol Occup Physiol. 1990;61:294–301. doi: 10.1007/BF00357616. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy J.D., Jones N.L. Arterialized capillary blood gases in exercise studies. Med Sci Sports. 1975;7:312–315. doi: 10.1249/00005768-197500740-00014. [DOI] [PubMed] [Google Scholar]
- 29.Rowell L.B. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 30.Jorfeldt L. Metabolism of L(plus)-lactate in human skeletal muscle during exercise. Acta Physiol Scand Suppl. 1970;338:1–67. [PubMed] [Google Scholar]
- 31.Bangsbo J., Aagaard T., Olsen M. Lactate and H+ uptake in inactive muscles during intense exercise in man. J Physiol. 1995;488(Pt 1):219–229. doi: 10.1113/jphysiol.1995.sp020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watt P.W., Gladden L.B., Hundal H.S. Effects of flow and contraction on lactate transport in the perfused rat hindlimb. Am J Physiol. 1994;267:E7–E13. doi: 10.1152/ajpendo.1994.267.1.E7. [DOI] [PubMed] [Google Scholar]