Abstract
With so much emphasis on reducing attrition and becoming more efficient in the delivery of healthcare, there are many opportunities to leverage existing clinical data in drug development and to foster the practice of reverse translation. The application of quantitative approaches to convert clinical trial and real‐world data to knowledge will continue to drive innovation. Herein we discuss recent examples of reverse translation and consider future opportunities to capture critical clinical knowledge to inform decision‐making in drug development.
In solving a problem of this sort, the grand thing is to be able to reason backward. That is a very useful accomplishment, and a very easy one, but people do not practice it much.
—Sherlock Holmes, A Study in Scarlet
BACKGROUND
The formation of interdisciplinary translational science groups within academic institutions, biopharmaceutical companies, regulatory agencies, and the healthcare industry illustrates the critical nature of these efforts. Translational science aims to convert the findings from basic science into meaningful therapeutic options or improved medical practice for patients. Much of the emphasis to date has been in the translation of benchside experimental data to the clinic. However, an area of great potential is the expansion of patient insights and real‐world data to inform clinical trials. Hence, reverse translation completes the cycle of knowledge gain by capturing critical learnings from the clinic to inform the design and implementation of future clinical studies.
THE ORIGINS AND SCOPE OF REVERSE TRANSLATION
The founding father of reverse translation, William Heberden the elder, was a physician scientist in the 18th century.1 He recorded intricate observations of disease while attending his patients at their bedside, which ultimately helped to distinguish more serious diseases from lesser diseases. Within translational medicine, there is a clear recognition of the value of bedside to bench learnings to refine target selection, target validation, and predictive animal models to design better drugs with lower attrition rates. Both successes and failures can be an equally valuable substrate to continually grow our fundamental understanding of disease. Recently, an analysis of discovery and development strategies for six programs directed at amyloid‐β was conducted to facilitate reverse translation of the failed Alzheimer's disease clinical studies.2 The critique highlighted the challenge of demonstrating target engagement, leaving open the question of whether the pharmacological hypothesis was fully tested. These motivating examples illustrate the broad scope and potential for reverse translation to impact the science of drug development.
QUANTITATIVE TOOLS TO ENABLE REVERSE TRANSLATION
Reducing attrition rates of drug candidates across the stages of drug development is a goal for the pharmaceutical industry. While multiple causes can be identified for attrition of drug candidates, there remains little doubt that mechanistic insights are needed for gaining full understanding. Thus, various candidate nomination processes at each phase of development are implemented at pharmaceutical organizations to evaluate if the molecule has demonstrated sufficient merit to move forward in development. Milligan et al. illustrated the evolution of model‐based drug development (MBDD) to a fully implemented concept with its applications across the early discovery to late‐stage development in a clinical utilization setting.3 The authors shared various elements of MBDD from identifying the right pathway to the right patients and quantitative tools that can be utilized across each of these activities.
As shown in Figure 1, while forward translation occurs in a traditional manner from bench to bedside, we propose that reverse translation does not necessarily have to follow this pattern. Using quantitative tools that are currently available, each of the various stages of drug development can utilize relevant internal and external data from clinical safety and efficacy data for appropriate decision‐making. This knowledge transfer from clinic to bench provides opportunity for utilizing clinical data for optimizing target engagement and therapeutic index during discovery and early clinical studies, set the target for clinical qualification for proof‐of‐concept studies, and define the right patient population and dose for efficacy at the clinical development stage. Each of the above learnings can be extremely valuable for the research and development organizations at a stage where there is an increasing expectation to accelerate drug development. Furthermore, some therapeutic areas such as oncology have noticed rapid development of highly efficacious immunotherapies with a wealth of clinical data generated in the recent past, thus making seamless reverse knowledge integration even more crucial.4 Insights gained from probing the relationship between genetics and epigenetics and drug response to define optimal oncology combination therapies may further enhance the demand for quantitative reverse translation in real time during drug development.
Figure 1.
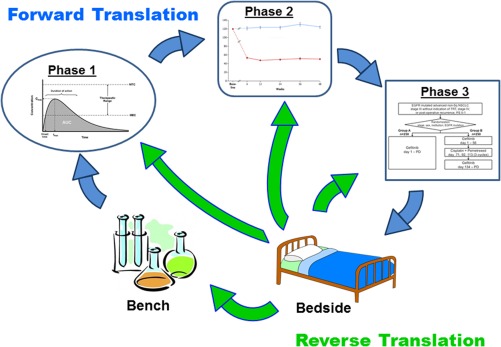
Schematic flow diagram of the cyclical nature of translational activities in support of clinical drug development.
KNOWLEDGE INTEGRATION USING QUANTITATIVE TOOLS
A central principle in the application of clinical pharmacology revolves around the concept of optimizing risks and benefits to achieve better outcomes in patients. The cornerstone in our ability to judge the success of these efforts revolves around the judicious application of quantitative methods. Many times, to achieve a greater understanding of the clinical pharmacology attributes, the application of reverse translational approaches has led to valuable insights that have enabled the success of clinical programs. Similar questions arise throughout the course of drug development that can be useful to inform forward translational strategies. Broad categories of these types of questions are provided in Supplemental Table 1.
Traditional pharmacokinetic/pharmacodynamic (PK/PD) modeling approaches can facilitate reverse translation and have been applied to help inform clinical study design. During the translation of animal data to humans, a common question revolves around the predictive value of in vitro data and animal models for anticipating drug response in humans. Modeling response data from animals and humans, adjusting for pharmacological differences in target binding and physiologic differences, represents a consistent opportunity for reverse translation. The modeling results define a rational strategy to integrate preclinical and clinical data for existing therapies into a knowledge‐based framework for application to new therapeutics. Disease progression modeling that relies on a longitudinal biomarker and outcome data can help define the quantitative relationships between early markers and later‐stage outcomes to aid clinical drug development. For example, in oncology models to relate tumor volume changes to patient survival have been developed. The value for reverse translation lies in the ability of such models to facilitate trial design using clinical trial simulations fueling the forward translation of future drug candidates. Model‐based meta‐analysis can be used to characterize the existing therapies in terms of their dose–response and longitudinal effects on clinical endpoints, focusing on summaries of aggregate data rather than individual patient information. Recently, aggregate clinical trial data for alogliptin, saxagliptin, sitagliptin, and vildagliptin were collected from publicly available data sources.5 A model‐based meta‐analysis demonstrated a universal relationship between DPP‐4 inhibition and HbA1c reduction after accounting for the placebo response and patient covariates. The model can be utilized to optimize the dose, duration of therapy, and patient population for future trials. Given the data‐intensive nature of reverse translation, it is exciting to consider the potential impact of real‐world data to define patient factors that contribute to the optimal application of therapeutics.
Quantitative systems pharmacology (QSP) is a rapidly expanding area that promises to build on our mechanistic understanding of disease pathways and merge with what has been the traditional focus of PK/PD studies. QSP platforms strive to integrate available in vitro, animal, and clinical data representing our existing knowledge and scientific understanding of the available data, and act as a central mechanism to achieve reverse translation of the available existing therapies and attributes of disease progression. While still a developing field, QSP has the potential to evolve into an application to interrogate disease hypotheses and facilitate our ability to implement successful drug development strategies leveraging reverse translation. Similarly, physiologically based pharmacokinetic (PBPK) modeling approaches represent another tool to facilitate reverse translation of quantitative clinical information. PBPK models consist of distinct elements that represent the human body (or system) and drug. The system model acts as a data repository of our cumulative knowledge of human physiology as it pertains to drug absorption, distribution, and elimination processes, including organ blood flows, volumes, drug‐metabolizing enzyme, and transporter expression. An advantage of PBPK and systems modeling platforms is that they are extensible, and can be updated as we learn more from ongoing and future studies.
FUTURE OPPORTUNITIES AND CHALLENGES
The goal of drug development remains to lower attrition of drugs while bringing efficacious medications more quickly to patients. Successful implementation of early clinical and translational research has proven to be an effective strategy, with successful proof‐of‐concept studies being the benchmark of success. Over the last decade, MBDD has evolved from a concept to a now commonly implemented strategy across major pharmaceutical institutions. The basic tenet of this concept is to integrate knowledge across the stages of drug development and inform decision‐making. Because the knowledge flow has to go back from bedside to bench, appropriate knowledge sharing across various functional teams is paramount to the successful application of translational sciences.
There are several steps that can enhance the value of reverse translation. First, appropriate platforms are required that can foster multiple data types for seamless integration of quantitative knowledge from bedside to each of the preceding development steps. Second, to embed expectations from pharmaceutical R&D governance bodies for reverse translation to aid forward translation. Third, to proactively engage with regulatory authorities to share quantitative aspects from reverse translation to achieve greater alignment across constituents.
CONCLUSION
To achieve the full impact and value of translational sciences, further integration of quantitative clinical pharmacology into the reverse translation cycle is required to optimize drug development. Opportunities remain to leverage the learnings from the bedside and implement them during clinical development to bring much needed therapeutic innovations to patients faster and more efficiently.
ACKNOWLEDGMENTS
The authors thank Karthik Venkatakrishnan, PhD, for thoughtful ideas at the ASCPT Annual Meeting. In addition, we thank Megan A. Gibbs, PhD, for critical review and thoughtful suggestions.
CONFLICT OF INTEREST
The authors are employed by AbbVie Inc. and may hold AbbVie stock and/or stock options.
Supporting information
Supplemental Table 1. Examples of the application of reverse translation to optimize clinical drug development.
References
- 1. Edelman, E.R. & LaMarco, K. William Heberden and reverse translation. Sci. Transl. Med. 7, 1–3 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Karran, E. & Hardy, J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 76, 185–205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milligan, P.A. et al Model‐based drug development: a rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 93: 502–514, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Venkatakrishnan, K. & Ecsedy, J.A. Enhancing the value of clinical pharmacodynamics in oncology drug development: an alliance between quantitative pharmacology and translational science. Clin. Pharmacol. Ther. 101, 99–113 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Gibbs, J.P. et al Quantitative model of the relationship between dipeptidyl peptidase‐4 (DPP‐4) inhibition and response: meta‐analysis of alogliptin, saxagliptin, sitagliptin, and vildagliptin efficacy results. J. Clin. Pharmacol. 52, 1494–1501 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Examples of the application of reverse translation to optimize clinical drug development.


