Abstract
Study and clinical testing of adult multipotent stromal cells (MSCs) are central to progressive improvements in veterinary regenerative medicine. Inherent limitations to long‐term culture preclude use for storage. Until cell line creation from primary isolates becomes routine, MSC stasis at cryogenic temperatures is required for this purpose. Many protocols and reagents, including cryoprotectants, used for veterinary MSCs are derived from those for human and rodent cells. Dissimilarities in cryopreservation strategies play a role in variable MSC behaviors. Familiarity with contemporary cryopreservation reagents and processes is essential to an appreciation of their impact on MSC survival and post‐cryopreservation behavior. In addition to these points, this review includes a brief history and description of current veterinary stem cell regulation.
1. INTRODUCTION
Adult multipotent stromal cells (MSCs) are increasing as standard therapy for a multitude of diverse pathologic conditions. Isolation of adequate cells for several therapeutic doses with minimally invasive tissue harvest is a perpetual struggle.1 Cell dosage varies widely among applications and is not established for any single treatment.2 Specific MSC immunophenotype subpopulations require extensive culture expansion due to low cell numbers,3 and genetic alterations and contamination risk increase with culture time.4, 5 In addition to allogeneic immunogenicity concerns,6 MSC quality varies with age and health status.7 Thermally dependent metabolic processes do not occur below −120°C, so MSCs are in metabolic stasis at liquid nitrogen temperature, around −196°C.8 Cell aliquots can be maintained for later administration immediately upon revitalization or after short‐term expansion.9 Cryopreservation also increases MSC availability as frozen cells can be delivered over long distances.10 Despite prevalent MSC cryopreservation, relatively little focus has been directed toward cell effects.
There is a growing awareness of differences between fresh and cryopreserved MSCs.11, 12, 13 Most veterinary MSC cryopreservation techniques are derived from human and murine protocols12, 14 and use cryopreservation medium that contains cryoprotectants (CP) and exogenous serum.15 Cells are cooled to about −80°C before transfer to liquid nitrogen.16 For revitalization, cells are thawed and then rinsed prior to culture.8 Each step, as well as cryopreservation duration, can impact MSC survival and attributes (Figure 1, Tables 1 and 2).
Figure 1.
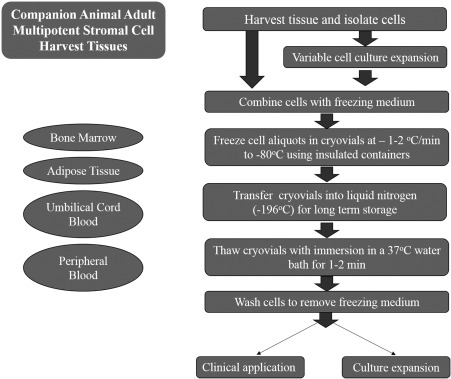
Schematic of adult multipotent stromal cell cryopreservation
Table 1.
Adult canine multipotent stromal cell freezing medium components, conditions, and post‐cryopreservation behaviors
| Harvest tissue | Passage | Cell aliquot (cells/mL) | Freezing medium | Freezing rate | Cooling process | Thawing process | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Adipose | 0 | ∼2 × 106 | DMEM, 10% FBS, 10% DMSO | −1°C/min | Insulated container at −80°C for 24 hours before liquid nitrogen | N/A | N/A | 17 |
| 0 | 1 × 106 | Serum‐free medium, 80% FBS, 10% DMSO | −1°C/min | Insulated container at −80°C overnight before liquid nitrogen | 37°C water bath for 2‐3 min | None | 18, 19 | |
| 0 | N/A | Low glucose DMEM, 30% FBS, 5% DMSO | N/A | N/A | N/A | N/A | 20 | |
| 1 | 3 × 106 | 90% FBS, 10% DMSO | −1°C/min | Insulated container at −80°C for 1 week before liquid nitrogen | 37°C water bath for 1‐2 min followed 10% FBS, 90% DMEM wash | Lower proliferation and telomerase | 15 | |
| Bone marrow | 0 | 1.0 × 106 | 10% DMSO, 10% FBS, α‐MEM | −1°C/min | Insulated container at −80°C for 7 days | 37°C water bath for 1 min | Lower viability, proliferative capacity | 21 |
Abbreviations: α‐MEM, minimum essential medium eagle (alpha modification); DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; N/A, not applicable.
Table 2.
Adult equine multipotent stromal cell (MSC) freezing medium components, conditions, and post‐cryopreservation behaviors
| Harvest tissue | Passage | Cell aliquot (cells/mL) | Freezing medium | Freezingrate | Cooling process | Thawing process | Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Adipose | N/A | ∼5 × 105 | 20% FBS, 10% DMSO, DMEM | −1°C/min | −80°C before liquid nitrogen | 37°C water bath for 2 min 20% FBS, 70% DMEM wash | Decreased proliferation rate | 22 |
| Bone marrow | N/A | ∼1 × 106 | 10% FBS, 10% DMSO, DMEM | −1°C/min | Insulated container for 24 hours at −80°C before liquid nitrogen | N/A | N/A | 16 |
| ∼10 × 106 | 20% serum, 10% DMSO, α‐MEM or 95% serum, 5% DMSO | −1°C/min | Isopropyl alcohol container at −80°C for 24 hours before liquid nitrogen | 35°C water bath until ice gone | Lower MSC numbers | 23 | ||
| Peripheral blood | 2‐3 | 2 × 106 | 90% FBS, 10% DMSO | −1°C/min | Insulated container at −80°C for 1 week before liquid nitrogen | 37°C water bath for 1‐2 min 10% FBS, 90% DMEM wash | Lower proliferation rate | 14 |
| Umbilical cord blood | N/A | 1 × 106 | 10% DMSO, 70% FBS, DMEM | N/A | −20°C for 1 hour, −80°C overnight before liquid nitrogen | 37°C water bath for 3 min 60% FBS, 40% DMEM wash | Lower cell viability that decreased rapidly with passage | 24 |
Abbreviations: α‐MEM, minimum essential medium eagle (alpha modification); DMEM, Dulbecco's Modified Eagle's medium; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; N/A, not applicable.
2. FREEZING
The cell freezing rate must be fast enough to avoid solute and electrolyte imbalances that cause cell dehydration and damage and slow enough to prevent extracellular and intracellular ice crystal formation.25 Cryoprotectants reduce the freezing point of the medium, so the mixture of cells, medium, and CPs is a eutectic system because the combined freezing point is lower than the individual components. During the freezing process, fluid moves from lower solute concentrations in unfrozen cells into partially frozen medium while plasma membranes prevent entrance of extracellular ice crystals. Slow freezing permits fluid to move out of the cells at a rate that results in a balanced osmotic pressure between cells and medium by the time the medium freezes. If the rate is too slow, cells are fatally dehydrated or their plasma membranes irreversibly damaged.8 If the rate is too high, there is insufficient fluid migration to maintain the high solute concentration that prevents fatal cell freezing.26 Cooling at a rate of −1°C/min27 with microprocessor‐controlled freezers or freezing containers with a heat transfer interface (isopropyl alcohol or insulation) reportedly has minimal effect on MSCs.28 Slow freezing of sterile specimens within sealed vials also minimizes contamination risk.29 Limitations of this cooling mechanism include cell dehydration and membrane damage, intracellular ice formation, and exposure to CPs.8
Vitrification is a form of MSC cooling that involves extremely rapid (>–1,000°C/s) cooling of cells immersed in CPs within open storage vessels.30 The process prevents fluid crystallization but requires potentially cytotoxic CP concentrations.30 Samples must always be at a cryogenic temperature, and open containers are a potential source of contamination.31 Vitrification is rarely used for veterinary MSC cryopreservation, so this review is focused on slow cooling.
3. THAWING
Cells pass through a temperature range for ice crystal formation, −15°C to −60°C, during freezing and thawing.8 Rapid thawing, 90‐100°C/min, by immersion in a 37°C water bath is often employed to prevent fluid crystallization.32 Murine hematopoietic progenitor cell survival is higher when cells are thawed rapidly at 900°C/min versus slowly at 2°C/min.33 Recovery rates of human erythroid progenitor cells are the same when they are thawed at 37°C or 20°C.34 The ideal thawing rate prevents ice formation and prolonged exposure to CPs and likely varies among both cells and CPs. Freezing and thawing processes should be customized and consistently utilized for a given species and MSC type.
4. CRYOPROTECTANTS
Cryoprotectants prevent cell damage during freezing and thawing.35, 36 Formulation and concentration varies among species, MSCs, and cooling techniques.27, 36 Relatively low CP concentrations, 1‐2 molar, used for slow freezing are associated with toxicity that differs among cell types and increases with time, temperature, concentration, and metabolic activity.36 There are 2 major CP categories, cell membrane permeable and impermeable.36 Those with high permeability tend to be most cytotoxic.8 Combining permeable CPs like dimethyl sulfoxide (DMSO), ethylene glycol, methanol, propylene glycol, and dimethylacetamide with less permeable CPs like polyvinylpyrrolidone, hydroxyethyl starch (HES), polyethylene glycol, and dextran reduces permeable CP concentrations.8, 36 DMSO and fetal bovine serum (FBS) are among the most common CPs used for companion animal MSC cryopreservation.14, 37 The carcinogenic properties of DMSO38 and xenogeneic proteins in FBS may alter the cells and impact postimplantation behavior.12 Some functions and limitations of these as well as alternative CPs follow.
4.1. Dimethyl sulfoxide
One of the most popular CPs, DMSO, stabilizes cell proteins39 and displaces intracellular fluid to equilibrate intracellular and extracellular electrolyte concentrations.40 Protein stabilization is mediated via hydrophobic interactions between DMSO and positively charged proteins, including cell membrane phospholipids.39 Additionally, DMSO forms high energy hydrogen bonds with water molecules to prevent ice formation.41
DMSO can cause MSC chemical toxicity and osmotic shock.40, 42 Hydrophobic interactions that protect proteins during cryopreservation can also denature and deactivate them.43 Increasing DMSO concentrations (5%‐20%) in the freezing medium is associated with lower survival and increased apoptotic gene expression in porcine bone marrow‐derived stromal cells (BMSCs).13 Neurotoxicity can occur from 10% DMSO, a typical concentration in cryopreservation medium.44 Serious side effects including hypotension and anaphylactic shock have been attributed to DMSO in cell suspensions administered intravenously to humans.44 Washing cells after thawing to reduce DMSO concentration results in cell loss and lower colony forming units,45 and complete DMSO removal is complex and time consuming.46 These points, among others, support continued efforts to identify replacements for DMSO in cryopreservation medium.
4.2. Fetal bovine serum
FBS collected at different gestational stages47 is a common culture medium ingredient that provides growth factors, nutrients, and hormones for cell proliferation and adhesion.40 It is also thought to act as a CP through protection of cell proteins and stabilization of osmotic pressure.40, 48 In addition to ethical, zoonosis, and xenogeneic protein concerns, variation in composition among FBS lots contributes to inconsistent cell culture performance.47 A recent finding that cryopreserved canine adipose‐derived multipotent stromal cells (ASCs) have increased CD44 expression compared to fresh cells was attributed to FBS in the freezing medium.12 The US Food and Drug Administration (FDA) does not permit use of FBS in products intended for humans or animals owing to potential immunogenicity.49 Autologous and allogeneic serum in MSC freezing media reportedly compares favorably to FBS for MSC viability, morphology, and plasticity.23 A study to assess different media effects on equine BMSCs included 2 freezing media composed of 20% serum, 10% DMSO, 70% DMEM or 95% serum and 5% DMSO, both with autologous serum, commercial pooled equine serum, or FBS.23 There was no difference in post‐thaw cell viability, morphology, or growth kinetics among different freezing media, and 95% autologous serum with 5% DMSO was recommended for short‐term (2‐3 days) cryopreservation.23 Serum‐free MSC cryopreservation medium has been shown to have similar or superior post‐cryopreservation outcomes compared to FBS‐containing media.48, 50 Increasing availability of FBS‐free freezing media may be important to improving consistency in MSC pre‐cryopreservation and post‐cryopreservation characteristics.
4.3. Impermeable cryoprotectants
Methylcellulose (MC) is a high molecular weight polymer in MSC freezing medium.27, 50 Human ASC post‐thaw cell viability with freezing medium containing MC in Dulbecco's modified Eagle's medium (DMEM) is greater than DMEM alone, but lower than that containing 80% serum, 10% DMSO, and 10% DMEM.35 Another popular CP is polyvinylpyrrolidone (PVP), a nontoxic, high molecular weight polymer.51, 52 The concentration of dissolved PVP increases in extracellular fluid as ice forms at −10°C to −20°C to create an osmotic gradient that draws fluid out of cells.53 Despite lower intracellular fluid, intracellular ice formation may occur at low PVP levels, and high concentrations of 20%‐40% can cause excessive cellular dehydration, cell necrosis, and membrane damage.51, 52 Human ASC viability and plasticity appears to be maintained with 10% PVP.51 HES is a synthetic polymer CP that absorbs water molecules (0.5 g water per 1 g HES) and maintains them in a solid state without crystallization during the cooling process.54 The polymer, widely used as a plasma volume substitute, is metabolized by glycolytic enzymes in vivo, so it does not have to be removed from thawed cells.54 A “6&5 solution” of physiologic saline, 6% HES, 5% DMSO, and 4% human serum albumin appears to maintain better viability, recovery rates and plasticity of human peripheral blood progenitors, cord blood stem cells, peripheral blood cells, and BMSCs compared to 10% DMSO in Roswell Park Memorial Institute 1640 medium.54 Similar findings are reported for canine BMSCs cryopreserved in the same solution.55 Novel cryopreservation solutions that support cell stasis without impacting inherent features will continue to promote availability and standardization of cell therapies across species.
5. CRYOPRESERVATION OF MESENCHYMAL STEM CELLS FROM DIFFERENT SPECIES
Human and veterinary MSCs are typically identified by cell surface antigens (Tables 1 and 2, Figure 2). Based on a recent review of FDA Investigational New Drug submissions for MSC‐based products, the top 7 surface antigens proposed as criteria for lot identity and purity are CD45, CD105, CD90, CD73, CD34, CD14, and the human leukocyte antigen class II (HLA Class II).56 Most submissions use a subset of the markers to confirm MSC identity. According to current International Society for Cellular Therapy standards, MSCs from bone marrow and adipose tissue have shared and distinct surface antigens.57 Over 70% of MSCs from both tissue sources are positive for CD13.58 The majority of BMSCs are CD45 positive versus less than 2% of ASCs. Most ASCs express CD73, CD90, and CD105, and 2%‐30% of BMSCs are positive. Surface antigen criteria are equally variable in veterinary species. Use of human MSC antigen panels in animals has been limited by species‐specific antigens.59 This is exemplified by a report that showed differences in surface antigen expression in fresh equine MSCs from bone marrow and adipose tissue determined with human‐specific antibodies and potential expression detected with real time polymerase chain reaction (RT‐PCR).60 At present, there are no established criteria for MSCs in any veterinary species. Identification of a panel of surface markers for universal MSC characterization will contribute to reliable and safe clinical translation.
Figure 2.
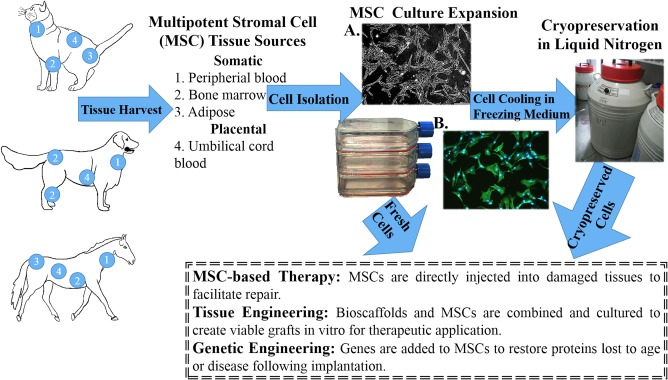
Schematic of companion animal adult multipotent stromal cell (MSC) processing. A, Polarized light photomicrograph of canine adipose‐derived multipotent stromal cells cultured in stromal medium (P2), 5×. B, Fluorescent photomicrograph of cells in A with cytoskeleton (actin, green) and nuclear (DNA, blue) staining, 5×
Some of the earliest cryopreservation descriptions were for spermatozoa, ova, and embryos.61 Cell cryopreservation has expanded with the evolution of cell‐based regenerative medicine. It is increasingly apparent that cryopreservation should be customized for species and cell type, especially with established differences among immunophenotypes in primary cell isolates.12, 15 Despite numerous variables, conflicting outcomes and inconsistent cryopreservation effects, common findings have emerged and are summarized below.
Comparisons among fresh and cryopreserved cells provide important information about storage and behavior to guide therapeutic and research applications and assessments. Existing literature documents similar, although not identical, changes in cell plasticity and expansion potential across species. Canine ASCs from subcutaneous and intra‐articular adipose tissues have lower sex determining region Y‐box 2 (SOX2) protein expression and distinct ultrastructure and immunophenotype compared to fresh ASCs following 30 days of cryopreservation in 80% FBS, 10% DMSO, and 10% DMEM.12 Two reports indicate that while canine ASCs and BMSCs maintain their fibroblast‐like morphology, alkaline phosphatase (ALP) activity, and plasticity following cryopreservation, there are significant differences from fresh cells.15, 21 Specifically, after 12 months of cryopreservation in freezing medium composed of 90% FBS and 10% DMSO, canine ASCs have lower proliferation and telomerase activity than fresh cells,15 and, separately, canine BMSCs have lower viability and proliferative capacity following 7 days of cryopreservation in freezing medium with 10% DMSO and 10% FBS.21 In contrast to these studies, another found lower ALP activity and plastic adhesion in canine BSMCs cryopreserved for 1 month.62 Equine peripheral blood MSCs cryopreserved in 90% FBS and 10% DMSO have faster proliferation but lower telomerase activity and myogenic plasticity compared to unfrozen cells.14 The in vitro osteogenic differentiation of fresh and cryopreserved rat, lapin, and porcine ASCs are reportedly similar on direct comparison.63 Human ASCs have lower proliferation and adipocytic and osteoblastic plasticity following cryopreservation in 90% FBS and 10% DMSO, and cryopreserved cells less effectively enhance calvarial healing in an athymic mouse model compared to fresh.11 These findings support the need to definitively characterize cell isolates before and after cryopreservation and to establish and maintain cryopreservation procedures for consistency.
Another finding common among species is more rapid loss of progenitor cell expansion and multipotentiality with passage and changes in surface antigen expression following cryopreservation. Canine BMSCs cryopreserved in 80% FBS, 10% DMSO, and 10% DMEM for 1 month had lower fibroblastic and osteoblastic colony forming unit frequencies than fresh cells with increasing passage.18 In one report, fresh canine P1 and P3 ASCs had significantly higher expression of CD29 than the same passages after cryopreservation in 80% FBS, 10% DMSO, and medium, and fresh cells had lower percentages of P1 ASCs that expressed CD44.12 Notably, CD29 and CD44 expression decreased with increasing passage more rapidly in cryopreserved versus fresh cells.12 In contrast to the former report, canine ASCs cryopreserved in 90% FBS and 10% DMSO were reported have similar expression of CD29, CD44, CD140a, CD117, CD34, and CD45 as fresh cells in cell passages P3 to P6, although cryopreserved cells had slower proliferation.15 Feline ASCs cryopreserved in identical freezing medium for 1 month had lower CD9 and CD105 expression compared to fresh cells, and the proliferation rate and osteoblastic capability decreased to a greater extent with increasing passage in cryopreserved versus fresh cells.1 The cell proliferation rate of equine ASCs frozen in 20% FBS, 70% DMEM‐high glucose, and 10% DMSO significantly declined at P12 while fresh cells did not show a similar decline until P15.22 Separately, the expression of CD90 and CD44 remained high after cryopreservation of equine peripheral blood MSCs but CD13 expression decreased slightly (61%) from that of fresh cells (78%).14 The potential aging effects of cryopreservation on MSCs that contribute to more rapid waning of cell expansion and plasticity compared to fresh cells is an important area of continued discovery to anticipate both in vitro and in vivo cell potential.
6. CELL PASSAGE NUMBER, CONCENTRATION, AND CRYOPRESERVATION DURATION
The amount of cell expansion prior to and duration of cryopreservation is an established factor in post‐cryopreservation cell traits. There is a direct relationship between equine umbilical cord blood MSC viability and pre‐cryopreservation passage number following cryopreservation in 20% DMEM, 70% FBS, and 10% DMSO for 8 weeks. Cell viability decreases from about 80.4% for P1 to 51.2% for P10.24 A study to assess cooling rate, end temperature, hold time, and thawing rate on human ASC cell membrane integrity showed a significant effect of thawing rate on only P3 and P4 while the interaction between cooling rate and end temperature was significant for P0‐P4.27
Results of studies to investigate effects of cell concentration on post‐cryopreservation viability vary. Human dental pulp MSCs cryopreserved in aliquots of 0.5, 1, 1.5, and 2 × 106 cells/mL in 10% DMSO and 90% medium had an average post‐thaw viability of 93%.64 In contrast, human adult ASC aliquots of 0.5 × 106 cells/mL cryopreserved in 10% DMSO, 10% medium, and 80% FBS had higher post‐cryopreservation viability than 0.25, 1, or 2 × 106 cells/mL.65 It is possible that cells are damaged from in adequate expansion space during the freezing process at high cell concentrations.65
The impact of cryopreservation duration may be most detectable following short‐term storage. Human BMSCs reportedly maintain trilineage differentiation capacity after 7 years of cryopreservation.32 However, total cell recovery is reportedly significantly lower after 5 (80%) versus 1 (90%) months of cryopreservation in 10% fetal calf serum, 10% DMSO, and 30% bovine serum albumin.66 These findings convey the importance of consistent cell expansion and aliquot concentration as well as consideration of the cryopreservation period when preparing MSCs for potential clinical application.
7. CELL TRANSPORTATION
Cell delivery from current Good Manufacturing Processes facilities to patient administration sites requires maintenance of frozen cells for variable time periods despite external temperature fluctuations.10 Vitrified cells are transported at cryogenic temperatures in dry shippers with liquid nitrogen in absorbent materials to avoid sample contact with liquid.46 Slow cooled samples can be shipped frozen in approved, polystyrene containers.67 Cryovials are often wrapped with precooled, absorbent material, and placed in a leak‐proof, biohazard grade container to prevent direct contact of samples with dry ice placed on top of the container within the polystyrene shipping box. Good practices include permanently labeled samples, inclusion of clear guidelines for sample handling and administration, a detailed inventory, signage on shipping containers, filing of all necessary shipping manifests, and package tracking.46 Contemporary travel makes it possible to transport cryopreserved samples globally, but national and international laws and regulations must be observed.
8. REGULATION OF CRYOPRESERVED REGENERATIVE CELLS FOR VETERINARY USE
Regulation of veterinary medicine in the United States has a complicated history with some products regulated by the US Department of Agriculture and others by the Food and Drug Administration Center for Veterinary Medicine (CVM). The Federal Food, Drug, and Cosmetic Act of 1938 established federal government regulation of animal health products. Veterinarians were allowed to prescribe human drugs and extra‐label use of veterinary drugs for animals under specific circumstances by the Animal Medicinal Drug Use Clarification Act of 1994. The Animal Drug Availability Act added moderation to the animal drug approval process, including flexible labeling and more direct communication between drug sponsors and the FDA in 1996. During early adult stem cell discoveries in 2002, the FDA announced a current good manufacturing practice initiative to create focus on the greatest public health risks of manufacturing procedures and ensure that process and product quality standards did not impede innovation. In 2004, passage of the Minor Use and Minor Species Animal Health Act encouraged development of treatments for species that may otherwise attract little interest. Collectively, these laws provided veterinarians reasonable discretion and freedom to use emergent drugs and medical devices. There was no specific guidance surrounding development and use of regenerative cell therapies for veterinarians before 2015.
The Guidance for Industry Publication #218, Cell‐Based Products for Animal Use, was published by the CVM in June 2015 to clarify regulation of cell therapies. Nonprimary cells that are culture expanded and intended to treat patients other than the donor are considered to be “drugs” and must go through FDA drug licensing and approval processes.68 Autologous cryopreserved cells to treat injury or disease in the donor are classified as either type I or type II cell‐based products (Table 3). Both are regulated as drugs and must undergo all indicated safety and efficacy testing and receive CVM authorization for use similar to allogeneic cell therapies. For type II classification, autologous cells must be minimally manipulated, for homologous use and for nonfood‐producing animals. They cannot be combined with anything other than water, crystalloids or sterilizing, preserving, or storage agents that do not raise additional safety concerns, or combined with or modified by a drug or a device. Prior to the advent of stem cell products, the term “type II autologous cells” was generally understood to mean whole or fractionated peripheral, umbilical cord, or marrow‐derived blood cells intended for transplantation, cells within cryopreserved mesenchymal tissues like fat, bone, ligament, and tendon, cartilage grafts, or β cell pancreatic islets.
Table 3.
Autologous cell‐based therapy classification
|
Autologous type I cell therapy criteria (one must be true) |
Autologous type II cell therapy criteria (all must be true) |
|---|---|
|
More than minimally manipulated (Extended period of in vitro culture) |
Minimally manipulated (Centrifugation only) |
| For nonhomologous use | For homologous use |
| For use in a food‐producing animal | For use in nonfood‐producing animals |
| Effects dependent on metabolic activity of cells | No statement regarding metabolic activity |
| Manufacture involves combination of cells with another article (Except water, crystalloids, or a sterilizing, preserving, or storage agent with no new product safety concerns) | Manufacture does not involve combination of cells with another article (Except water, crystalloids, or a sterilizing, preserving, or storage agent with no new product safety concerns) |
Improved methods of cell preservation with ingredients beyond serum and DMSO complicate cell classification since commercial cryopreservation solution components may be considered drugs under some circumstances. Use of cryopreserved cells in combination with popular blood derivatives like platelet rich plasma occupies a nebulous area in the classification scheme. The speed of discovery in the stem cell arena exceeds development of regulations governing their use. Similarly, definitions of “homologous use” and “minimally manipulated” do not entirely capture current knowledge of stem cell functionality. Academic and industrial scientists continue to work with regulatory authorities to achieve and maintain contemporary language that is consistent with intended and practical use.
In summary, CVM consultation should be sought prior to manufacture and use of cryopreserved cells as a commercial treatment, especially since cells transported across state lines are automatically within federal regulatory jurisdiction. Additionally, state requirements for reporting and licensing a Good Tissue Practice cell banking facility must be observed. Use of cell processing and banking services provided by veterinary regenerative medicine companies appears to be acceptable as the long the provider has implemented appropriate quality and safety standards.
9. CONCLUSION
Cryopreservation of adult MSCs is central to their development, availability, and use. The practice is relatively new in veterinary medicine, and its use will continue to grow.35, 52 It is clear that fresh and frozen MSCs are not identical, although differences are not fully established. Efforts to discover and standardize cryopreservation protocols based on species, tissue, and cryostasis duration will continue to advance therapeutic efficacy and safety of cryopreserved cells.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to this report.
ACKNOWLEDGMENT
Graduate student stipend support provided by the Grayson Jockey Club Research Foundation and the Louisiana State University Equine Health Studies Program.
Duan W, Lopez MJ, Hicok K. Adult multipotent stromal cell cryopreservation: Pluses and pitfalls. Veterinary Surgery. 2018;47:19‐29. https://doi.org/10.1111/vsu.12730
Funding information Grayson Jockey Club Research Foundation; Louisiana State University Equine Health Studies Program
REFERENCES
- 1. Zhang N, Dietrich MA, Lopez MJ. Therapeutic doses of multipotent stromal cells from minimal adipose tissue. Stem Cell Rev Rep. 2014;10:600‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez‐Silos V, Camacho‐Morales A, Fuentes‐Mera L. Mesenchymal stem cells subpopulations: application for orthopedic regenerative medicine. Stem Cells Int. 2016;2016:3187491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capes‐Davis A, Theodosopoulos G, Atkin I, et al. Check your cultures! A list of cross‐contaminated or misidentified cell lines. Int J Cancer. 2010;127:1‐8. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Zhang Z, Chi Y, et al. Long‐term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4:e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641‐1655. [DOI] [PubMed] [Google Scholar]
- 7. Zaim M, Karaman S, Cetin G, et al. Donor age and long‐term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175‐1186. [DOI] [PubMed] [Google Scholar]
- 8. Gao D, Critser J. Mechanisms of cryoinjury in living cells. ILAR J. 2000;41:187‐196. [DOI] [PubMed] [Google Scholar]
- 9. Yong KW, Wan Safwani WKZ, Xu F, et al. Cryopreservation of human mesenchymal stem cells for clinical applications: current methods and challenges. Biopreserv Biobank. 2015;13:231‐239. [DOI] [PubMed] [Google Scholar]
- 10. Harel A. Cryopreservation and cell banking for autologous mesenchymal stem cell‐based therapies. Cell Tissue Transplant Ther. 2013;5:1‐7. [Google Scholar]
- 11. James AW, Levi B, Nelson ER, et al. Deleterious effects of freezing on osteogenic differentiation of human adipose‐derived stromal cells in vitro and in vivo. Stem Cells Dev. 2010;20:427‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan W, Lopez MJ. Effects of cryopreservation on canine multipotent stromal cells from subcutaneous and infrapatellar adipose tissue. Stem Cell Rev Rep. 2016;12:257‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ock S‐A, Rho G‐J. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cell Transplant. 2011;20:1231‐1239. [DOI] [PubMed] [Google Scholar]
- 14. Martinello T, Bronzini I, Maccatrozzo L, et al. Cryopreservation does not affect the stem characteristics of multipotent cells isolated from equine peripheral blood. Tissue Eng Part C Methods. 2010;16:771‐781. [DOI] [PubMed] [Google Scholar]
- 15. Martinello T, Bronzini I, Maccatrozzo L, et al. Canine adipose‐derived‐mesenchymal stem cells do not lose stem features after a long‐term cryopreservation. Res Vet Sci. 2011;91:18‐24. [DOI] [PubMed] [Google Scholar]
- 16. Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2011;21:273‐283. [DOI] [PubMed] [Google Scholar]
- 17. Screven R, Kenyon E, Myers MJ, et al. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 2014;161:21‐31. [DOI] [PubMed] [Google Scholar]
- 18. Spencer ND, Chun R, Vidal MA, Gimble JM, Lopez MJ. In vitro expansion and differentiation of fresh and revitalized adult canine bone marrow‐derived and adipose tissue‐derived stromal cells. Vet J. 2012;191:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G. Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue–harvesting site. Cell Biol Int. 2013;37:789‐798. [DOI] [PubMed] [Google Scholar]
- 20. Reich CM, Raabe O, Wenisch S, Bridger PS, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue‐and bone marrow‐derived mesenchymal stem cells–a comparative study. Vet Res Commun. 2012;36:139‐148. [DOI] [PubMed] [Google Scholar]
- 21. Edamura K, Nakano R, Fujimoto K, Teshima K, Asano K, Tanaka S. Effects of cryopreservation on the cell viability, proliferative capacity and neuronal differentiation potential of canine bone marrow stromal cells. J Vet Med Sci. 2014;76:573‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mambelli LI, Santos EJ, Frazao PJ, et al. Characterization of equine adipose tissue–derived progenitor cells before and after cryopreservation. Tissue Eng Part C Methods. 2009;15:87‐94. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell A, Rivas KA, Smith R III, Watts AE. Cryopreservation of equine mesenchymal stem cells in 95% autologous serum and 5% DMSO does not alter post‐thaw growth or morphology in vitro compared to fetal bovine serum or allogeneic serum at 20 or 95% and DMSO at 10 or 5%. Stem Cell Res Ther. 2015;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eini F, Foroutan T, Bidadkosh A, Barin A, Dehghan MM, Tajik P. The effects of freeze/thawing process on cryopreserved equine umbilical cord blood‐derived mesenchymal stem cells. Comp Clin Path. 2012;21:1713‐1718. [Google Scholar]
- 25. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology. 1977;14:251‐272. [DOI] [PubMed] [Google Scholar]
- 26. Mazur P. Equilibrium, quasi‐equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys. 1990;17:53‐92. [DOI] [PubMed] [Google Scholar]
- 27. Thirumala S, Zvonic S, Floyd E, Gimble JM, Devireddy RV. Effect of various freezing parameters on the immediate post–thaw membrane integrity of adipose tissue derived adult stem cells. Biotechnol Prog. 2005;21:1511‐1524. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama WM, Thompson ML, Ehrhardt RO. Cryopreservation and thawing of cells. Curr Protoc Immunol. 2012;5. Appendix 3:3G. [DOI] [PubMed] [Google Scholar]
- 29. Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24:2457‐2467. [DOI] [PubMed] [Google Scholar]
- 30. Fowler A, Toner M. Cryo‐injury and biopreservation. Ann N Y Acad Sci. 2005;1066:119‐135. [DOI] [PubMed] [Google Scholar]
- 31. Taylor M, Song Y, Brockbank K. Vitrification in tissue preservation: new developments In: Fuller BJ, Lane N, Benson EE, eds. Life in the Frozen State. Boca Raton, FL: CRC Press; 2004:604‐641. [Google Scholar]
- 32. Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leibo S, Farrant J, Mazur P, Hanna MG Jr, Smith LH. Effects of freezing on marrow stem cell suspensions: interactions of cooling and warming rates in the presence of PVP, sucrose, or glycerol. Cryobiology. 1970;6:315‐332. [DOI] [PubMed] [Google Scholar]
- 34. Katayama Y, Yano T, Bessho A, et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant. 1997;19:283‐287. [DOI] [PubMed] [Google Scholar]
- 35. Thirumala S, Gimble JM, Devireddy RV. Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum‐free freezing media for cryopreservation of adipose‐derived adult stem cells. Stem Cells Dev. 2010;19:513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters. 2004;25:375‐388. [PubMed] [Google Scholar]
- 37. Marx C, Silveira MD, Beyer Nardi N. Adipose‐derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 2015;24:803‐813. [DOI] [PubMed] [Google Scholar]
- 38. Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anchordoguy TJ, Cecchini CA, Crowe JH, Crowe LM. Insights into the cryoprotective mechanism of dimethyl sulfoxide for phospholipid bilayers. Cryobiology. 1991;28:467‐473. [DOI] [PubMed] [Google Scholar]
- 40. Liu Y, Xu X, Ma X, Martin‐Rendon E, Watt S, Cui Z. Cryopreservation of human bone marrow‐derived mesenchymal stem cells with reduced dimethylsulfoxide and well‐defined freezing solutions. Biotechnol Prog. 2010;26:1635‐1643. [DOI] [PubMed] [Google Scholar]
- 41. Weng L, Li W, Zuo J, Chen C. Osmolality and unfrozen water content of aqueous solution of dimethyl sulfoxide. J Chem Eng Data. 2011;56:3175‐3182. [Google Scholar]
- 42. Scheinkönig C, Kappicht S, Kolb HJ, Schleuning M. Adoption of long‐term cultures to evaluate the cryoprotective potential of trehalose for freezing hematopoietic stem cells. Bone Marrow Transplant. 2004;34:531‐536. [DOI] [PubMed] [Google Scholar]
- 43. Fahy GM, Lilley TH, Linsdell H, Douglas MS, Meryman HT. Cryoprotectant toxicity and cryoprotectant toxicity reduction: in search of molecular mechanisms. Cryobiology. 1990;27:247‐268. [DOI] [PubMed] [Google Scholar]
- 44. Junior A, Arrais C, Saboya R, Velasques RD, Junqueira PL, Dulley FL. Neurotoxicity associated with dimethyl sulfoxide‐preserved hematopoietic progenitor cell infusion. Bone Marrow Transplant. 2008;41:95‐97. [DOI] [PubMed] [Google Scholar]
- 45. Fry L, Querol S, Gomez S, McArdle S, Rees R, Madrigal JA. Assessing the toxic effects of DMSO on cord blood to determine exposure time limits and the optimum concentration for cryopreservation. Vox Sang. 2015;109:181‐190. [DOI] [PubMed] [Google Scholar]
- 46. Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002;30:219‐227. [DOI] [PubMed] [Google Scholar]
- 48. Verdanova M, Pytlik R, Kalbacova MH. Evaluation of sericin as a fetal bovine serum‐replacing cryoprotectant during freezing of human mesenchymal stromal cells and human osteoblast‐like cells. Biopreserv Biobank. 2014;12:99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble JM. Immunogenicity of allogeneic adipose‐derived stem cells in a rat spinal fusion model. Tissue Eng Part A. 2009;15:2677‐2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Freimark D, Sehl C, Weber C, et al. Systematic parameter optimization of a Me 2 SO‐and serum‐free cryopreservation protocol for human mesenchymal stem cells. Cryobiology. 2011;63:67‐75. [DOI] [PubMed] [Google Scholar]
- 51. Thirumala S, Wu X, Gimble JM, Devireddy RV. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue‐derived adult stem cells. Tissue Eng Part C Methods. 2009;16:783‐792. [DOI] [PubMed] [Google Scholar]
- 52. Guha A, Devireddy R. Polyvinylpyrrolidone (PVP) mitigates the damaging effects of intracellular ice formation in adult stem cells. Ann Biomed Eng. 2010;38:1826‐1835. [DOI] [PubMed] [Google Scholar]
- 53. McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978;15:382‐390. [DOI] [PubMed] [Google Scholar]
- 54. Stolzing A, Naaldijk Y, Fedorova V, Sethe S. Hydroxyethylstarch in cryopreservation–mechanisms, benefits and problems. Transfus Apher Sci. 2012;46:137‐147. [DOI] [PubMed] [Google Scholar]
- 55. Ide K, Matsuura S, Fujino Y, Ohno K, Tsujimoto H. Investigation of various methods for the cryopreservation of canine bone marrow‐derived CD34 + cells. J Vet Med Sci. 2008;70:1211‐1217. [DOI] [PubMed] [Google Scholar]
- 56. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC‐based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141‐145. [DOI] [PubMed] [Google Scholar]
- 57. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 58. Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Schauwer C, Meyer E, van de Walle GR, van Soom A. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 2011;75:1431‐1443. [DOI] [PubMed] [Google Scholar]
- 60. Ranera B, Lyahyai J, Romero A, et al. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011;144:147‐154. [DOI] [PubMed] [Google Scholar]
- 61. Slade NP, Takeda T, Squires EL. Short report: cryopreservation of the equine embryo. Equine Vet J. 1985;17:40‐40. [DOI] [PubMed] [Google Scholar]
- 62. Li H, Yan F, Lei L, Li Y, Xiao Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs. 2008;190:94‐101. [DOI] [PubMed] [Google Scholar]
- 63. Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose‐derived stem cells: from small to large animal models. Cell Tissue Res. 2009;338:401‐411. [DOI] [PubMed] [Google Scholar]
- 64. Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp‐derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose‐derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322‐324. [DOI] [PubMed] [Google Scholar]
- 66. Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow‐derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453‐463. [DOI] [PubMed] [Google Scholar]
- 67. Hunt CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. 2011;38:107‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fink DW. FDA regulation of stem cell‐based products. Science. 2009;324:1662‐1663. [DOI] [PubMed] [Google Scholar]


