Abstract
Oestrogen receptors (ERs) and β‐adrenergic receptors (βARs) play important roles in the cardiovascular system. Moreover, these receptors are expressed in cardiac myocytes and vascular tissues. Numerous experimental observations support the hypothesis that similarities and interactions exist between the signalling pathways of ERs (ERα, ERβ and GPR30) and βARs (β1AR, β2AR and β3AR). The recently discovered oestrogen receptor GPR30 shares structural features with the βARs, and this forms the basis for the interactions and functional overlap. GPR30 possesses protein kinase A (PKA) phosphorylation sites and PDZ binding motifs and interacts with A‐kinase anchoring protein 5 (AKAP5), all of which enable its interaction with the βAR pathways. The interactions between ERs and βARs occur downstream of the G‐protein‐coupled receptor, through the Gαs and Gαi proteins. This review presents an up‐to‐date description of ERs and βARs and demonstrates functional synergism and interactions among these receptors in cardiac cells. We explore their signalling cascades and the mechanisms that orchestrate their interactions and propose new perspectives on the signalling patterns for the GPR30 based on its structural resemblance to the βARs. In addition, we explore the relevance of these interactions to cell physiology, drugs (especially β‐blockers and calcium channel blockers) and cardioprotection. Furthermore, a receptor‐independent mechanism for oestrogen and its influence on the expression of βARs and calcium‐handling proteins are discussed. Finally, we highlight promising therapeutic avenues that can be derived from the shared pathways, especially the phosphatidylinositol‐3‐OH kinase (PI3K/Akt) pathway.
Keywords: β‐adrenergic receptors, cardioprotection, crosstalk, GPR30, intracellular signalling, oestrogen receptors
1. INTRODUCTION
The risk of cardiovascular diseases (CVDs) is higher in aged women compared to that of pre‐menopausal women.1 In addition, development of CVDs in men occurs at a relatively young age, while the risk of CVDs in women accelerates after menopause.1 These observations are attributed, in part, to gender‐related cardioprotective roles of oestrogen. Moreover, numerous studies have described the expression of oestrogen receptors (ERs) in various tissues of the cardiovascular system (CVS). There are 3 classes of ERs: the ERα, ERβ and the G‐protein‐coupled receptor 30 (GPR30). All these ERs are expressed in the heart cells and in the vascular vessels.2, 3, 4, 5 Each receptor subtype shows variation in function and in tissue‐specific expression. Based on ligand specificity, oestrogen and some of its metabolic intermediate products activate the ERs triggering both genomic and non‐genomic actions.6 Results from several experiments have implicated oestrogen in the chronotropic and inotropic functions of the heart,7, 8, 9 and in cardiac perfusion.10, 11, 12
In their recent study, Debortoli et al.10 showed that activation of GPR30 modulated coronary circulation by regulating coronary perfusion pressure in rats. Accordingly, left ventricular diastolic dysfunction is predominant in post‐menopausal women.13 Consistent with these reports, Giraud et al.14 used Magness et al.'s menopause model15 and showed that left ventricle diameters and end‐diastolic volume were elevated by chronic oestrogen replacement in this model. In the ovine model, 3 research groups showed that 17β‐oestradiol administration increased coronary blood flow significantly.15, 16, 17, 18, 19, 20 Collectively, they showed that the pattern of rises in coronary perfusion is independent of patterns of rises in cardiac output. They also noted that the pattern of raises in cardiac output was graded, that is a 30‐ to 60‐min delay followed by an increase and a plateau at 90‐120 minutes, a phenomenon observed in reproductive tissues such as uterus and mammary gland.15, 20 Mershon et al. confirmed that these effects of oestrogen are ER‐dependent as they were prevented by pre‐treatment with antagonist ICI‐182 780.18
The heart rhythm and contraction are mainly regulated by the sympathetic nervous system (SNS), via the β‐adrenergic receptors (βARs) that connect and convey the SNS signals to the heart. βARs are divided into β1AR, β2AR and β3AR.21 Interestingly, signalling pathways of ERs are intertwined with those of the βARs pointing to the possibility of functional convergence in modulating the physiology of the CVS. In fact, crosstalk between ERα and α1b‐adrenergic receptor was reported previously.22, 23 Furthermore, we and others established that oestrogen alters gene expression of βARs and calcium (Ca2+)‐handling proteins of the CVS.24, 25, 26 Proteins that regulate cardiac Ca2+ include the Na+/Ca2+ exchanger pump (NCX), L‐type Ca2+ channel (LTCC), phospholamban (PLB), sarcoplasmic reticulum Ca2+‐ATPase (SERCA) and ryanodine receptors (RyRs; Figure 1).27 Consequently, the effects of oestrogen on the Ca2+‐handling proteins have direct implications on the contractile machinery of the myocardium.
Figure 1.
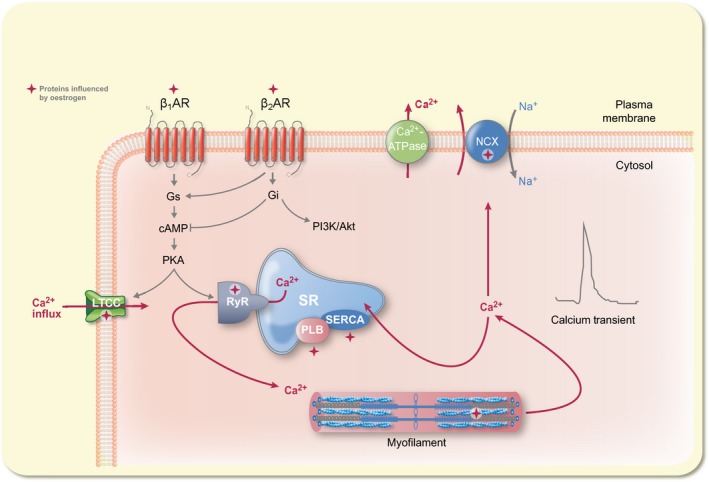
Cardiac Ca2+‐handling proteins and Ca2+ trafficking in cardiomyocyte regulated by βARs and oestrogen. Illustration of a network of calcium‐handling proteins and Ca2+ trafficking in cardiomyocyte which are activated and regulated by βARs and oestrogen. Purple arrows indicate movement of Ca2+. The symbol  indicates points at which oestrogen exerts influence on cardiac contractile function. Abbreviations: LTCC, L‐type channel; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; SERCA, sarcoplasmic reticulum Ca2+‐ATPase; NCX, Na+/Ca2+ exchanger pump; PLB, phospholamban
indicates points at which oestrogen exerts influence on cardiac contractile function. Abbreviations: LTCC, L‐type channel; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; SERCA, sarcoplasmic reticulum Ca2+‐ATPase; NCX, Na+/Ca2+ exchanger pump; PLB, phospholamban
In recent decades, the need to understand the cardiovascular functions of oestrogen and βARs has received much interest from researchers. The discovery of GPR30,28 which shares structural features with βARs, has expanded the functional scope of oestrogen. In this regard, it is important to re‐evaluate the relationships between ER and βAR signalling pathways and their interdependence in modulating the cardiovascular physiology. This review focuses on the recent experimental studies to describe the roles and mechanisms of ERs and βARs. We firstly discuss their classification, functions and the basis of cardiac physiology. We then provide novel illustrations on the points of integration between oestrogen and adrenergic signalling pathways. Our aim is to provide evidence for the hypothesis that there are interactions and functional cooperation between ER and βAR signalling pathways, particularly in the heart. We highlight the therapeutic potential of the interactions and explore their implications on the postulated cardioprotection conferred by oestrogen, β‐blockers and Ca2+ channel blockers. We also discuss the ERs and βARs as coregulators of cardiac Ca2+‐handling proteins.
2. A RECAP OF β‐ADRENERGIC RECEPTORS IN THE CARDIOVASCULAR SYSTEM
2.1. βAR‐specific features
βARs are members of the G‐protein‐coupled receptors (GPCRs) that classically form 7 transmembrane loops, with extracellular and intracellular terminals. Three βAR subdivisions, β1AR, β2AR and β3AR, are encoded by different genes.21 Moreover, the 3 receptors are expressed in the plasma membrane in the CVS,29 as well as in the cardiac nuclear envelope of adult rats and mouse myocytes for β1AR and β3AR.30, 31, 32In heart myocytes, the number of β1ARs is higher than that of β2ARs, while β3ARs show the least abundance.33 βARs are linked to heterogeneous intracellular signalling pathways and proteins. In addition, their expressions vary under physiological and pathological conditions.34
2.2. βAR‐specific signalling
βARs are activated by noradrenaline and adrenaline released from the SNS and adrenal glands respectively. However, once activated, the βARs trigger diverse intracellular pathways.35 β1ARs couple to the stimulatory unit of the G protein (Gαs) leading to the synthesis of cyclic adenosine monophosphate (cAMP) by adenylyl cyclase (AC) enzyme. On the other hand, β2ARs are pleiotropic receptors that couple to the Gαs, the inhibitory G protein (Gαi) and the Gβγ.36, 37 At the physiological state, β2ARs couple to the Gαs, whereas at high adrenaline concentration, they switch to Gαi, a phenomenon referred to as stimulus‐mediated trafficking.38, 39 Activation of the β2AR/Gαi pathway inhibits cAMP production, an opposing effect to β2AR/Gαs and β1AR/Gαs activation (Figure 1).
With regard to structure and function, the β3ARs display distinct differences to β1ARs and β2ARs. Notably, the cytoplasmic C‐terminus of the β3ARs lacks the target amino acid sequences for protein kinase A (PKA) and cardiac G‐protein‐coupled receptor kinase 2 (GRK2) phosphorylation.40, 41 Consequently, β3ARs are less susceptible to PKA/GRK2‐mediated receptor recycling and desensitization in response to hyperstimulation.40 Two isoforms, β3aAR and β3bAR, were reported in Chinese hamster ovary (CHO) cells and 3T3‐L1 adipocytes.42, 43, 44, 45, 46 The β3aAR isoform coupled to the Gαi, whereas β3bAR coupled to both Gαs and Gαi. At present, there are no reports regarding the existence of the 2 isoforms in human cardiac cells. β3AR has largely been associated with metabolic functions. Nevertheless, β3AR stimulation induced positive inotropy in human atrial cells,47 but it had no effect on the inotropy of human ventricular cells.48 β3AR may influence chronotropic functions through the nitric oxide (NO)/guanosine 3′,5′‐monophosphate (cGMP) pathway.49 Activation of plasma β3AR‐NO synthase/guanylyl cyclase pathway was shown to influence the nuclear β3AR‐mediated gene transcription, suggesting a crosstalk between the surface and nuclear β3ARs.50
2.3. The basis of the heart's function
βARs mediate the SNS regulation of the cardiac functions.51 These functions are primarily orchestrated by activation of β1ARs, which constitute up to 80% of the entire cardiac βAR density of healthy human, and to a lesser extent by the β2ARs.33, 35 Moreover, β2ARs have a higher affinity for adrenaline, while β1ARs have almost equal affinities for both noradrenaline and adrenaline.39 On the other hand, activation of the β3ARs is largely associated with negative inotropy during catecholaminergic stress.47
Once activated, βARs initiate cAMP synthesis by coupling to the Gαs, a GTP‐binding protein. This cAMP, in turn, activates PKA. What follows is the induction of intracellular rise in Ca2+ transients via the tightly regulated network of ion channels. PKA‐mediated inotropic effects are orchestrated through the phosphorylation of 2 main channels: the LTCC located in the T‐tubular network formed by sarcolemmal membrane invaginations and the RyR2 receptors on the SR membrane. Phosphorylation of LTCC allows Ca2+ entry as inward current.52 These Ca2+ currents further stimulate Ca2+ release from SR, the intracellular stores, by the opening of RyR2 receptors which are also phosphorylated by PKA. This phenomenon is referred to as calcium‐induced calcium release. The resultant Ca2+ transient activates the myofilament protein troponin C turning on cardiomyocyte contraction. The size of Ca2+ transients is a key determinant of the strength of the contraction.27 PKA also regulates cardiac relaxation by phosphorylating phospholamban (PLB), a modulator of SERCA. In its unphosphorylated state, PLB inactivates SERCA. This effect is reversed following PLB phosphorylation which permits Ca2+ uptake back to the SR by SERCA. In addition, sarcolemmal Na+/Ca2+ exchanger pump (NCX) accelerates Ca2+ extrusion, which together with SR Ca2+ uptake diminishes the Ca2+ transient resulting in relaxation.
Besides the classical cAMP/PKA pathway, cAMP also acts through the recently described intracellular protein named exchange protein directly activated by cAMP (EPAC).53 Classified into EPAC1 and EPAC2, these proteins bind cAMP and function as guanine exchange factors (GEFs) for Ras superfamily. The EPAC pathway amplifies the cardiovascular functions of β1AR/cAMP and provides alternative modulation of βAR activation. Indeed, both EPAC1 and EPAC2 are present in cardiomyocytes.53 Diverse physiological roles of EPAC proteins have been recently reviewed by Lezoualc'h et al.54 Activation of EPAC was linked to ventricular hypertrophy, vasorelaxation and in the regulation of Ca2+ through RyR and PLB phosphorylation,54 indicating synergism between the cAMP/PKA and cAMP/EPAC pathways.
Another downstream target of β1AR activation is the multimeric protein Ca2+/calmodulin kinase II (CaMKII).55 Activation of this kinase indirectly relies on the PKA‐mediated rise in cytosolic Ca2+ and intracellular levels of calmodulin.56 Recent findings reveal that CaMKII activation augments the LTCC current and increases the RyR open probability57 and phosphorylation of PLB,58 showing its participation in cardiac contractility. CaMKII has also been associated with detrimental effects including apoptosis, necroptosis and arrhythmias.59
3. CLASSIFICATION, LOCALIZATION AND DISTRIBUTION OF ERS IN THE CVS
The cardiovascular functions of oestrogen are mediated by ERs. These cellular receptors are categorized as nuclear receptors (ERα and ERβ), which modulate transcription of specific gene sets, and membrane‐bound receptor (GPR30, also known as GPER1), which mediates rapid, non‐genomic actions of oestrogen. ERs are expressed in cardiomyocytes,3 cardiac fibroblasts4 and VSMCs;5 however, their expression and cellular locations are not fully understood. For instance, Pugach et al.3 reported that ERβ was not expressed in either neonatal or adult male or female mouse or rat ventricular myocytes. This observation is inconsistent with earlier reports.60, 61, 62 In addition, there are controversies surrounding the cellular localization of GPR30. In particular, some researchers reported that GPR30 was nearly confined to the endoplasmic reticulum in COS cell lines,63 while others observed both cytosolic and membrane localization in HEK293 cells64 and in rat VSMCs.65 The differences in these reports may be related to tissue‐specific variations. It is also important to note that oestrogen is a lipophilic hormone that crosses the plasma membrane to access the intracellular receptors. Therefore, both membrane and subcellular localization of GPR30, as observed, are conceivable as they are accessible to oestrogen.
With regard to gender, a study carried out on VSMCs of rats showed that GPR30 expression was similar in both males and females.65 However, gender differences with regard to ERα and ERβ expression were also reported. Whereas the mRNA levels of ERα were equivalent in hearts of both men and women,3, 66 ERβ had greater expression in males than females in both healthy and diseased human hearts.67 However, these observations are in contradiction to another report that showed an opposite expression pattern where ERβ expression was not different in male and female cardiomyocytes, while ERα expression varied with gender.68 Elsewhere, ERα and ERβ protein levels in male and female rabbit hearts were not different.69 Further studies are advocated to reconcile these findings.
In addition, Ma et al.65 observed that subcellular location of ERα was not influenced by its activation; a similar observation was reported for GPR30 in a subsequent study.64 However, change in subcellular location of the ERs may vary as in heart failure. In healthy hearts, ERα was localized to the intercalated disc, while in failing hearts, its location shifted away from the intercalated discs.66 This implies that cardiomyopathies may influence the subcellular localization and by extension the signalling of the ERs. Moreover, oestradiol supplementation in ovariectomized (OVX) rats increased ERα and ERβ protein levels.70 Variation in relative abundance of the ERs was recently reported. Quantitative real‐time PCR analysis of male mouse ventricle found that GPR30 mRNA levels were thrice those of ERα and 17‐fold greater than those of ERβ.62 Different genes located on different chromosomes encode each ER subtype. While alternative splicing of the gene transcripts leads to multiple subtypes of ERα and up to 5 described transcripts of ERβ,71 GPR30 only exists in 1 isoform.3, 6, 72 The distinct features of the ER subtypes are outlined in Table 1. Taken together, expression of the ER subtypes in the cardiovascular system remains contentious with regard to tissue‐specific expression. Discrepancies from the previous reports could be due to the methods used or species of tissue investigated. Further investigations are required to resolve the inconsistencies.
Table 1.
Features and classification of oestrogen receptors
| Receptor features | GPR30 | ERα | ERβ |
|---|---|---|---|
| Cellular location | Plasma membrane96 | Nucleus83, 169 | Nucleus130 |
| Cytosol64 | Cytosol60, 130 | Cytosol60 | |
| Plasma membrane130, 170 | Plasma membrane82, 169 | ||
| Onset of physiological effects | Rapid actions (effects within seconds to minutes)102 | Rapid and genomic action (effects within minutes to days)82, 102, 171, 172, 173, 174 | Rapid and genomic action (effects within minutes to days)82, 83, 173, 174, 175 |
| Genetics | GPER gene located on chromosome 7p22.372 | ESR1 gene located on chromosome 6q25.1176 | ESR2 gene located on chromosome 14q23.2178 |
| No introns, 1 isoform72 | 8 exons, 3 isoforms176 | 8 exons, 5 isoforms178, 179 | |
| Protein size 375 amino acids72 | Protein size 595 amino acids177 | Protein size 530 amino acids178 | |
| Cardiovascular tissue distribution | Cardiac fibroblasts4 | Cardiac fibroblasts2 | Cardiac fibroblasts2 |
| Vascular tissues180 | Vascular tissues93, 102 | Vascular tissues93 | |
| Cardiomyocytes62 | Cardiomyocytes2, 3 | Cardiomyocytes (unresolved) | |
| Ligands | E262 | E2130 | E283 |
| G‐1181 | PPT76 | DPN76 | |
| Relative abundance in cardiac cells | Highest62 | Low62 | Lowest62 |
E2, 17β‐oestradiol; PPT, propylpyrazoletriol; DPN, propylpyrazoletriol; G‐1, GPR30 agonist; ERα, oestrogen receptor α; ERβ, oestrogen receptor β; GPR30, G‐protein‐coupled oestrogen receptor 30.
4. ER ACTIVATION, SIGNALLING PATHWAYS AND CELL FUNCTIONS
4.1. ER activation
Similar to other steroids hormones, oestrogen signalling is initiated by the binding of 17β‐oestradiol or xenoestrogens73 and oestradiol metabolites74, 75 to ER. Synthetic receptor‐specific agonists with selective binding affinities have also been developed: propylpyrazoletriol (PPT) for ERα, diarylpropionitrile (DPN) for ERβ and G1 for GPR30.76 Noteworthy, each receptor subtype or isoform displays different affinities to 17β‐oestradiol and other oestrogenic ligands.77, 78 Moreover, oestrogens are of different forms (estrone, oestradiol and estriol) which exist in a dynamic equilibrium in circulation. Considering that oestrogen activates multiple receptors, ER subtype–specific functions determine cellular responses to oestrogen stimulation. It has been postulated that the balance between oestrogen forms is responsible for activation of different signalling pathways under certain physiological conditions based on the premise that ERs possess different affinities for each oestrogen subtype.78 Furthermore, 17β‐oestradiol synthesis occurs through enzymatic modifications of precursors such as androgens by aromatase enzyme. Considering that aromatase is expressed within the heart,68 the possibility of cardiac oestrogen synthesis further augments the importance of oestrogen to the cardiovascular physiology in addition to circulating oestrogens. It is also likely that the adipose tissue surrounding the heart is the source of the C19 androgen conversion to C18 oestrogen, considering that epicardial fat covers up to 80% of heart's surface and constitutes 20% of heart's weight.79
4.2. Signalling pathways
4.2.1. Receptor‐mediated signalling: genomic vs non‐genomic pathways
Binding of oestrogen to its receptors (membrane or nuclear) triggers 2 types of cellular effects defined by the timing of onset. (i) Part of the effects occurs through the well‐established pathway of ER‐mediated transcription of certain genes. Conventionally, this pathway is known as a genomic pathway and occurs within hours to days.80 The ERα and ERβ receptors largely execute these genomic functions. Upon oestrogen binding, these ERs undergo conformational changes allowing nuclear translocation and dimerization of the oestrogen‐ER complex with oestrogen response elements, found at promoter areas of specific genes. Through this mechanism, oestrogen influences expression of cellular proteins. However, this pathway is not exclusive to nuclear receptors. Activation of membrane receptor GPR30 induced gene transcription.4 (ii) Another pathway that emerges after oestrogen binding is the non‐genomic pathway. This pathway requires activation of several different signalling cascades that alter cellular functions of proteins and ion channels. Most of these actions occur within seconds or minutes and are regulated by ERα and GPR30.7, 62, 81 There are reports indicating the presence of ERβ in the cytosol and plasma membrane and that they are responsible for rapid non‐genomic signalling in endothelial cells. It is yet to be established whether ERβ exists on the plasma membrane of adult cardiomyocytes.60, 82, 83, 84 In addition, crosstalk between membrane ERs and nuclear ERs has been reported.85 There is growing interest to decipher mechanisms that underlie non‐genomic oestrogen signalling. How the non‐genomic oestrogen pathways integrate with βAR pathways forms the basis of the discussion dealt with in Section 3 of this article. Moreover, the interaction of ER signalling with adrenergic receptor pathways was observed between the ERα and α1b‐adrenergic receptors.22
4.2.2. Receptor‐independent signalling
Besides the conventional receptor‐mediated mechanisms of oestrogen, experimental observations have hinted at the possibility of an alternative mechanism that does not involve membranous or nuclear ERs.81, 86, 87 This mechanism falls in the category of rapid and non‐genomic pathways and does not involve oestrogen‐receptor binding. A previous experiment showed that oestrogen induced negative inotropy in ERα and ERβ knockout mouse cardiomyocytes and its inhibition of the LTCC current was not altered from wild‐type myocytes.88 The same laboratory later demonstrated that oestrogen directly interacts with the LTCC protein and inhibits LTCC current even at resting state on cultured HEK293 cells.86 Indeed, similar observations have been reported for a broad range of ion channels (see review89). Research on the rapid non‐genomic roles of oestrogen has been primarily focused on the membranous receptors. Therefore, the observation that oestrogen could bind directly to ion channels introduces a reclassification of its mechanism of actions and adds to the growing debate on its non‐genomic functions.
4.3. Tissue‐specific functions
Oestrogen plays several functions in the CVS. Here, we highlight some of the cell‐specific roles of oestrogen without much detail because of the limitation defined by the purpose of this review. Activation of GPR30 inhibited proliferation of rat cardiac fibroblasts and collagen synthesis in both in vivo and ev vivo settings.4 These effects were attributed to oestrogen‐induced expression of cell cycle proteins and alterations in expression of matrix metalloproteinase‐12. GPR30 also mediates cardioprotection against ischaemia/reperfusion injury by improving the heart function, reducing infarct size, and mitochondrial Ca2+ overload.62 On the other hand, ERα agonists induced vasodilation on vascular smooth muscle cells of the aorta.90 In our previous study, we demonstrated that oestrogen and G1 decreased the expression of β1ARs and induced negative inotropy.24, 91 ERβ has also been reported to offer cardioprotection in cardiomyocytes.92 Together, these findings demonstrate that ERs are important effectors of oestrogen signals in cardiovascular tissues.
5. SIMILARITY IN MOLECULAR PATHWAYS OF ERS AND ΒARS
The crosstalk between ERs and βARs is a concept that was revealed from earlier studies.22, 23 Evidence from recent studies further recognizes oestrogen as a key hormone that influences the expression of βARs26, 91 and cardiac ion‐handling proteins.93, 94 Moreover, oestrogen regulates the cardiac contractile functions, which are otherwise under the control of adrenergic receptors (details discussed in Sections 4 and 4.1). Intriguingly, the structure (of GPR30) and signalling pathways of ERs are functionally closer/related to those of the βARs, at least partially.72, 93, 95, 96, 97, 98 Some of the cellular roles of ERs synergize or oppose the effects produced by βAR activation. Therefore, here we explore, side by side, the correlation among β1ARs, β2ARs and β3ARs vs. ERα, ERβ and GPR30. We discuss various points of integration between their signalling pathways. We note 3 main pathways along which these classes of receptors interact.
5.1. Signalling along the GPCR/Gαs/cAMP pathway
Similar to βARs, GPR30 possesses PKA phosphorylation sites and PDZ binding motifs and associates with A‐kinase anchoring proteins (AKAPs).64 In addition, GPR30 uniquely possesses 4 CaMKII binding sites unlike all other GPCRs.99 Furthermore, like the βARs, GPR30 couples to the classical GPCR proteins, Gαs 100, 101 and Gαi/o,63, 96, 102 in cardiovascular tissues (Figure 2). On this basis, activation of GPR30 partially mimics the signalling pathway of βARs with regard to its downstream cascades. Initial activation of β1AR, β2AR and GPR30 leads to coupling to Gαs protein.96, 103 Activation of Gαs triggers the production of cAMP by AC enzyme. Subsequently, PKA and EPAC amplify the cAMP signal. In coronary arteries, GPR30 was shown to activate this pathway including the production of PKA and EPAC proteins.101 The cAMP is hydrolysed by phosphodiesterases (PDEs) that determine the specificity of its signalling so as to avoid “off‐target” reactions through the creation of microdomains.104 This process occurs through the multimeric units formed between βAR/Gαs/AC and PDEs.104 In addition, it is known that PKA interacts with PDE4 and facilitates the degradation of cAMP by associating with the scaffold proteins AKAPs.105 Therefore, considering the observation that GPR30 signals through the Gαs/AC/cAMP pathway, it would be interesting to define whether the GPR30/Gαs/AC complex also participates in compartmentalization of cAMP signals in cardiac cells. The PKA generated downstream of GPR30 might interact with PDEs, under the direction of AKAP5, to regulate cAMP degradation as for β1ARs and β2ARs. Moreover, we speculate that GPR30's ability to activate EPAC might also influence β1AR/cAMP/EPAC‐mediated functions. Besides GPR30, activation of the ERα elevated PKA in VSMCs of aortic tissue further illustrating oestrogen involvement in the GPCR/Gαs/cAMP signalling pathway.102
Figure 2.
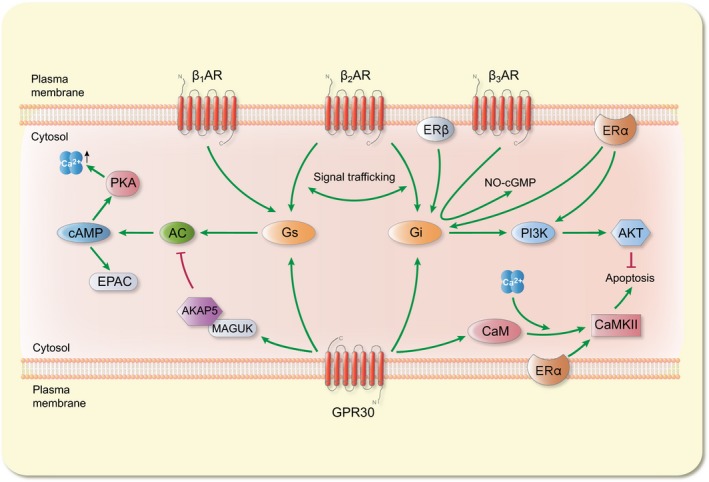
Interaction of oestrogen signalling and beta‐adrenergic signalling pathways. The symbol  represents cytosolic Ca2+ rise. The symbol
represents cytosolic Ca2+ rise. The symbol  represents inhibition signal. The symbol
represents inhibition signal. The symbol  represents activation signal. Signalling pathways of βARs (β1AR, β2AR and β3AR) and ERs (ERα, ERβ and GPR30) are integrated through the Gs and Gi pathways. Effector proteins PKA and EPAC affect the Gs‐cAMP signals. The resultant effects play crucial roles in cardiac contraction by increasing cytosolic Ca2+ levels. Alternatively, the receptors may activate the Gi, which mediates anti‐apoptosis signals through the PI3K/Akt pathway. Elevated cytosolic Ca2+ levels activate CaM and CaMKII, which induces apoptosis. GPR30 may inhibit the AC enzyme through the MAGUK/AKAP5 complex. GPR30: G‐protein‐coupled receptor 30; E2: 17β‐oestradiol; EPAC: exchange protein directly activated by cAMP; AKAP5: A‐kinase anchoring protein 5; MAGUK: membrane‐associated guanylate kinase; CaM: calmodulin; CaMKII: Ca2+/calmodulin kinase II
represents activation signal. Signalling pathways of βARs (β1AR, β2AR and β3AR) and ERs (ERα, ERβ and GPR30) are integrated through the Gs and Gi pathways. Effector proteins PKA and EPAC affect the Gs‐cAMP signals. The resultant effects play crucial roles in cardiac contraction by increasing cytosolic Ca2+ levels. Alternatively, the receptors may activate the Gi, which mediates anti‐apoptosis signals through the PI3K/Akt pathway. Elevated cytosolic Ca2+ levels activate CaM and CaMKII, which induces apoptosis. GPR30 may inhibit the AC enzyme through the MAGUK/AKAP5 complex. GPR30: G‐protein‐coupled receptor 30; E2: 17β‐oestradiol; EPAC: exchange protein directly activated by cAMP; AKAP5: A‐kinase anchoring protein 5; MAGUK: membrane‐associated guanylate kinase; CaM: calmodulin; CaMKII: Ca2+/calmodulin kinase II
Additional interactions are possible due to the structural resemblance between GPR30 and βARs. βARs interact with cellular proteins through PDZ motifs located at their C‐terminus regions. The PDZ motifs give a bearing on the localization and signalling of βARs. Of note, β1AR, β2AR and GPR30 possess type I PDZ binding motifs: ‐ESKV, ‐DSLL and ‐SSAV respectively.106, 107 PDZ domains recognize and bind to specific amino acid sequences of their target proteins.106 For instance, the β1AR (‐ESKV) motif was shown to be a determinant factor for its coupling to Gαs and not Gαi. Induced disruption of this motif permitted β1AR/Gαi coupling.106 On the other hand, β2AR (‐DSLL) motif plays a role in its coupling to Gαi.108 In comparison, the GPR30 PDZ motif (‐SSAV) was implicated in recycling and translocation of GPR30 in HEK293 cells,64 but it is not known whether this motif may influence GPR30's ability to couple to Gαs or Gαi. In addition to the PDZ motifs, β2AR phosphorylation by PKA influences its ability to bind Gαs or Gαi.39 Although GPR30 coupling to Gαs or Gαi may be dependent on cell/tissue type, collectively, these observations raise the possibility that PKA phosphorylation or disruption of its PDZ motif would have implications on its coupling to Gαs and Gαi as is the case for β1ARs and β2ARs. This possibility calls for further inquiry to the conditions under which GPR30 couples to Gαi and Gαs, and the proteins that interact with its PDZ motif.
One functional implication of this motif draws from the recent observation that GPR30 inhibited βAR‐mediated production of cAMP in response to isoproterenol stimulation in HEK293 cells.64 This inhibitory effect was dependent on a complex formed by GPR30, through its PDZ motif, with membrane‐associated guanylate kinases (MAGUKs) and AKAP5 (Figure 2).64 The primary role of AKAPs is to bind and regulate the subcellular location of PKA. Interestingly, the binding of AKAP5 to β1ARs facilitated its recycling by enhancing PKA phosphorylation of the receptor.109, 110, 111 Moreover, it is established that oestrogen regulates expression of β1ARs. Therefore, an association of GPR30 with AKAP5 and possibly other unidentified proteins could be a mechanism through which oestrogen participates in the regulation of β1AR density. Further research should be carried out to characterize the interactions of GPR30 with AKAPs and their implications on cellular functions.
Although β1AR, β2AR and GPR30 are capable of coupling to Gαs, their cellular effects are not similar. For instance, activation of β1ARs,112 ERα and GPR30113 triggers production of calmodulin and activation of CaMKII, while β2ARs and ERβ do not. Importantly, the observation that GPR30 activates cAMP through Gαs pathway challenges previous findings that oestrogen induced negative inotropy at both cardiomyocyte and organ levels. Therefore, the effects of GPR30/Gαs/cAMP pathway may not be identical to the classical βAR/Gαs/cAMP pathway, especially in cardiomyocytes. However, as we reported, oestrogen may tilt the activation of β2AR/Gαs or β2AR/Gαi pathways in certain disease conditions, as in stress‐induced cardiomyopathy.114
5.2. Signalling along the GPCR/Gαi/PI3K/Akt pathway
As mentioned earlier, GPR30, like β2ARs and β3ARs, couple to the Gαi subunit.96, 102, 115 Furthermore, GPR30 activates the phosphatidylinositol‐3‐OH kinase (PI3K)/Akt pathway resulting in inhibition of apoptosis through regulation of the Bcl‐2 family of proteins.24, 102, 115, 116 The GPR30/PI3K/Akt‐mediated cardioprotection against ischaemia/reperfusion injury in cardiomyocytes was orchestrated through upregulation of anti‐apoptosis Bcl‐2 protein and downregulation of pro‐apoptosis Bax protein.116 In accordance with our previous report, inhibition of β2ARs exposes cardiomyocytes to cell death in ischaemic conditions.117 The β2AR/Gαi pathway triggered anti‐apoptotic signals through the PI3K/Akt pathway.118 These findings present the evidence that PI3K/Akt cardioprotective pathway is shared by the GPR30 and β2ARs. In addition to the GPR30, oestrogen activates the ERα, which directly binds the p85 alpha regulatory component of the PI3K.119
In addition, PKA phosphorylation of the β2ARs enables switching of its coupling from Gαs to Gαi under extreme catecholamine stimulation,39 a phenomenon referred to as signal trafficking (Figure 1). In this context, β2ARs act as a switch that coordinates synthesis of cAMP and indicates cross‐communication between β1AR and β2AR signalling. Moreover, AKAP5 tethering of PKA allows it to phosphorylate β2ARs.120 Considering that GPR30 possesses PKA phosphorylation sites, we speculate that through AKAP5, PKA phosphorylation of GPR30 may influence its ability to activate Gαs or Gαi. Although we appreciate that such signal trafficking is intricately complicated and may involve different mechanisms, further research is necessary to determine whether this phenomenon can be replicated in adult cardiomyocytes. Unlike β2ARs, the conditions under which GPR30 activates Gαs or Gαi are not clearly understood. Perhaps a possible hint as to when GPR30 activates Gαi comes from the observation that under stress conditions, both β2ARs and GPR30 activate Gαi/PI3K/Akt pathway to confer cardioprotection.116, 118 However, we recognize that the requirements for GPR30 coupling to Gαs or Gαi in cardiomyocytes need further investigation.
Unlike β1ARs, β2ARs and GPR30, β3ARs lack PKA phosphorylation sites.40 Therefore, for β3ARs, there is a great deal of variability with respect to their ability to activate both Gαs and Gαi. Some β3AR splice variants were shown to display dual coupling to Gαs and Gαi in other cell types, although not in cardiac cells.121 β3ARs act through Gαi/o to suppress contractility via induction of NO‐cGMP pathway under chronic catecholaminergic stimulation.122 In addition, β3AR/NO/cGMP pathway was enhanced in the presence of β1AR blocker, which was interpreted to be beneficial in chronic volume‐overloaded heart.123 Based on the evidence presented above, the ER and βAR signalling pathways function as interdependent networks/partners whose roles have profound effects on the cardiovascular system. In summary, Gαs and Gαi act as pivots around which both ER and βAR signalling pathways converge. β2AR and GPR30 signal through the Gαs/AC/cAMP and Gαi/PI3K/Akt pathways in the cardiovascular system. Similarly, both GPR30 and ERα signalling cascades interact through the PI3K/Akt pathway (Figure 1). PI3K/Akt acts as a focal pathway that unifies GPR30‐, ERα‐ and β2AR‐mediated cardioprotection. In general, the Gαs/AC/cAMP and Gαi/PI3K/Akt pathways seem to trigger opposing effects. For example, the cAMP produced by βAR stimulation was shown to inhibit the activity of Akt kinase indicating an inverse relationship between the 2 pathways.124 Lastly, the net cellular effects of the interactions between ERs and βARs might be dependent on the cell/tissue type.
5.3. Localization of ERs and βARs to the caveolae
β1AR, β2AR and β3AR have been shown to signal and express in the caveolin‐rich fractions of the plasma membrane (Figure 3). Caveolin proteins are found in flask‐shaped subdomains of plasma membranes known as caveolae.125 β2AR localizes almost exclusively to caveolin 3‐rich membrane fractions of rat cardiomyocytes, while β1AR localizes to both caveolar and non‐caveolar membrane fractions.126 These spatial distributions play a role in the differential activation of cAMP signals by β1AR and β2AR.127 For instance, colocalization of β2AR with the Ca2+ channel LTCC and caveolin 3 is essential for its signalling and ability to invoke intracellular Ca2+,128 while caveolin 3 interaction with AC V acts as a scaffolding protein which participates in β1AR signals that induce LTCC current in ventricular cardiomyocytes.129 ERα was associated with eNOS activation in the caveolae of endothelial cells,130 while ERβ was found in the caveolae where it mediated eNOS signals.82 Strikingly, overexpression of β2AR enhanced vascular repair of endothelial progenitor cells in mice through the eNOS pathway.131 Therefore, localization of β2AR and ERβ in caveolae of vascular cells and their ability to signal through the eNOS pathway indicate functional cooperation between the 2 receptors. Caveolin 1 is a scaffold protein for both ERα and β3AR.132, 133 The association of β3AR with caveolin 1 was shown to govern its ability to couple to Gαi/o proteins in CHO‐K1 cells.133 On the other hand, it has been established that ERα and ERβ interact directly with Gαi and that these interactions occur in close proximity to the caveolae domains.134 The physiological relevance of the possible interactions among ERs, caveolins and βARs in adult cardiomyocytes remains to be fully established. Although there is a dearth of evidence regarding this view, the data sets reviewed here imply direct or indirect crosstalk among ERs and βARs. Further research is required to identify multilevel communications and interactions among these receptors.
Figure 3.
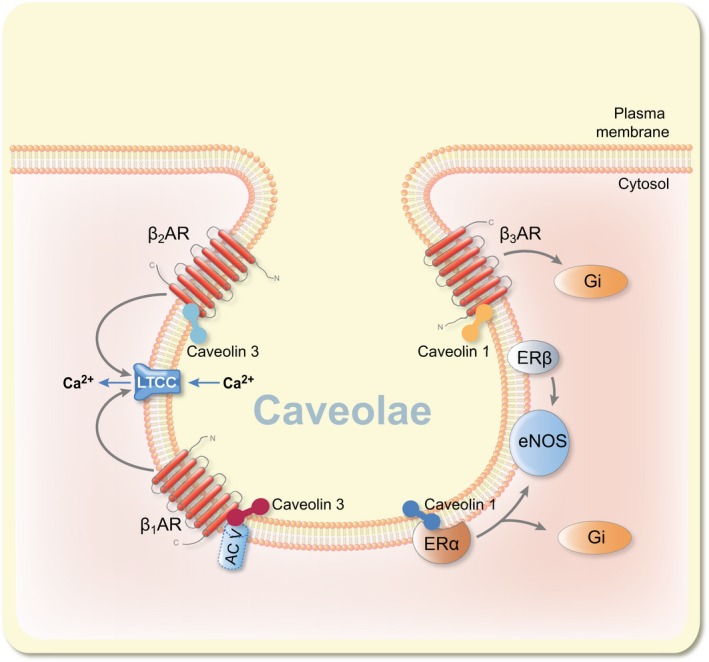
βAR and ER signalling through caveolae. In this view, β2AR associates with caveolin 3 and LTCC to transduce signals that increase cellular Ca2+. β1AR interacts with caveolin 3 and AC V to induce LTCC current. Caveolin 1 interacts with β3AR and governs its ability to couple to Gαi. ERα colocalizes with caveolin 1 and signals through Gαi and activates eNOS pathway. ERβ mediates the activation of eNOS by oestrogen in the caveolae. AC V: adenylyl cyclase V; ERα: oestrogen receptor alpha; ERβ: oestrogen receptor beta; eNOS: endothelial nitric oxide synthase; LTCC: L‐type calcium channel
A summary of the shared features is as follows:
β2ARs, β3ARs, ERα, ERβ and GPR30 couple to Gαi subunit.
β1ARs, GPR30 and ERα activate calmodulin/CaMKII.
β1ARs, β2ARs and GPR30 couple to Gαs subunit.
β2ARs, ERα and GPR30 trigger the PI3K/Akt pathway.
β3AR and ERα associate with caveolin 1, while β1AR and β2AR associate with caveolin 3.
6. OESTROGEN INFLUENCE ON THE EXPRESSION OF βARS
Expression of βARs in the cardiovascular vessels is influenced, in part, by age, by gender and by drugs targeting these receptors.135 The ratio of β1ARs, β2ARs and β3ARs may also vary with disease status.136 In pre‐menopausal women, the cardiac expression of β1ARs and β2ARs decreases with age until menopause after which it stabilizes.135 On the contrary, there is no significant association between age and β1AR/β2AR expression in men.135 Together with other numerous animal experiments, these observations seem to indicate that sex hormones, particularly oestrogen, play regulatory roles in the expression of βARs. Moreover, these roles may be due to the direct action of oestrogen on βAR signalling cascades or indirectly through adaptative responses to oestrogen environment.
6.1. β1AR expression
Our studies91 and others25, 137, 138 systematically showed that ovariectomy (OVX) increased the expression of β1ARs and induced negative inotropy in rat hearts subjected to ischaemia/reperfusion injury (I/R) and, in addition, that this role of oestrogen was mediated by the ERα.91 On the other hand, activation of GPR30 in ventricular myocytes from OVX rats reversed the effects of OVX on β1AR expression.24 Taken together, these findings indicate that oestrogen‐mediated influences on β1AR levels are affected by both ERα and GPR30. Furthermore, it was shown that oestrogen not only suppressed the expression of β1ARs but also increased sensitivity to catecholamines.139 Therefore, the downregulation of β1ARs by oestrogen may be a cardioprotective strategy against the adverse effects associated with hyperstimulation of β1ARs.
6.2. β2AR expression
In female rat models of I/R and heart failure, oestrogen, acting through the GPR3024 and ERα,91 increased the expression of β2ARs. We further showed that oestrogen in combination with testosterone enhanced the cardiac expression of β2ARs in OVX rats.140 Other researchers also reported that β2AR mRNA and protein were upregulated in female hearts but not male hearts in response to the arteriovenous fistula procedure.141 Taken together, these observations show that oestrogen decreases β1AR expression and upregulates β2AR expression in cardiac cells.
6.3. β3AR expression
Currently, information on direct effects of oestrogen on β3AR expression in cardiac tissues is lacking. However, variations in expression of β3ARs in adipose tissues have been linked to oestrogen levels. One group reported that oestrogen elevated the expression of β3ARs in murine adipocytes in culture,142 while another group observed that oestrogen decreased the quantity of β3ARs in brown adipose tissue of female rats in vivo.143 The discrepancies in these reports might be attributed to the methodologies used, that is real‐time PCR vs. radio‐ligand binding method used in the latter report or due to the inherent differences between in vitro and in vivo studies.
7. ERS AND βARS AS COREGULATORS OF CARDIAC CA2+‐HANDLING PROTEINS
Intracellular Ca2+ levels in cardiac cells are coregulated by both ERs and βARs. Numerous studies provide compelling evidence that oestrogen influences the expression levels of Ca2+‐handling proteins, whose functions are primarily under the regulation of βARs (Figure 1).144 In addition to the major proteins LTCC, RyR, PLB, SERCA and NCX,27 sarcolipin (SLN), an inhibitor of SERCA, plays a role in cardiac Ca2+ handling.145 However, to our knowledge, the influence of oestrogen on the expression or function of SLN has not been documented and hence needs to be clarified. The results of previous studies that were designed to investigate the effect of oestrogen on expression of cardiac Ca2+‐handling proteins channels are summarized in Table 2. In summary, the reports on oestrogen regulation of SERCA were largely consistent that oestrogen increased expression of SERCA, while its expression was decreased in OVX animal models92, 93, 146, 147, 148 (full reference list in Table 2). Similarly, oestrogen increased expression of NCX,26, 149, 150, 151 while it was decreased in OVX rats.26 However, in other studies, no change was observed in the expression of NCX expression in both oestrogen treatment and OVX animals.152, 153, 154 Although oestrogen decreased the expression of PLB,155, 156, 157 and OVX increased its expression,155, 156, 157, 158 no change in expression was found in other reports.26, 147, 153, 159, 160 On the other hand, oestrogen downregulated the RyR expression,161 while in other reports both OVX and oestrogen treatments had no effect on RyR expression.26, 153 Similarly, studies examining the role of oestrogen on the expression of LTCC yielded mixed results. Oestrogen decreased LTCC protein levels in rat ventricular myocytes (RVMs),26, 161 while OVX increased its expression in RVMs,26 but decreased its expression in mouse ventricular myocytes.154
Table 2.
Summary of previous studies designed to investigate the effects of oestrogens on cardiac Ca2+‐handling proteins
| Name of protein | Oestrogen effect | ER involved | Species/cell type/tissue | References | |
|---|---|---|---|---|---|
| Oestrogen | OVX | ||||
| L‐type channel | ↓ | ↑ | Not investigated | Rat ventricular tissue | 26 |
| ↑ | ERα | Rabbit heart | 69 | ||
| ↓ | Not investigated | Neonatal rat ventricular cells | 161 | ||
| ↓ | Not investigated | Mouse ventricle tissue | 154 | ||
| Ryanodine receptor | ─ | ─ | Not investigated | Rat ventricular tissue | 26 |
| ↓ | Not investigated | Neonatal rat ventricular cells | 161 | ||
| ─ | ─ | GPR30 | Rat left ventricle tissue | 153 | |
| SERCA | ↓ | Not investigated | Rat heart tissue | 158 | |
| ─ | ─ | Not investigated | Rat ventricular tissue | 26 | |
| ↑ | Not investigated | Mouse apical ventricle | 149 | ||
| ↑ | Not investigated | Zebrafish hearts | 182 | ||
| ─ | ─ | ERα | Rat ventricular cells | 159 | |
| ─ | Not investigated | Mouse ventricular tissue | 160 | ||
| ↑ | ERα and ERβ | Cultured murine cardiomyocytes | 146 | ||
| ↑ | ↓ | Not investigated | Rat ventricular tissue | 147 | |
| ↑ | ERβ | Mouse ventricle tissue | 92 | ||
| ↑ | Not investigated | Rat embryonic heart H9C2 | 148 | ||
| ↑ | ERα and ERβ | Pig coronary arteries tissue | 93 | ||
| ↑ | ↓ | GPR30 | Rat cardiac microsomes | 162 | |
| ─ | ─ | Not investigated | Rat heart tissue | 152 | |
| ↑ | Not investigated | Mouse ventricle tissue | 151 | ||
| ↑ | ↓ | Not investigated | Rat heart tissue | 155 | |
| ─ | ─ | Not investigated | Rat left ventricle tissue | 156 | |
| ─ | ─ | GPR30 | Rat left ventricle tissue | 153 | |
| ─ | Not investigated | Mouse ventricle tissue | 154 | ||
| ↑ | ↓ | Not investigated | Mouse ventricle tissue | 183 | |
| ↑ | ↓ | Not investigated | Rat left ventricle tissue | 157 | |
| Phospholamban | ↑ | Not investigated | Rat heart tissue | 158 | |
| ─ | ─ | Not investigated | Rat ventricular tissue | 26 | |
|
─ Male ↑Female |
Not investigated | Mouse ventricle tissue | 149 | ||
| ─ | ─ | ERα | Ventricular cells | 159 | |
| ─ | Not investigated | Mouse ventricular tissue | 160 | ||
| ─ | ─ | Not investigated | Rat ventricular tissue | 147 | |
| ↓ | Not investigated | Rat cardiac microsomes | 162 | ||
| ↓ | ↑ | Not investigated | Rat heart tissue | 155 | |
| ↓ | ↑ | Not investigated | Rat left ventricle tissue | 156 | |
| ─ | ─ | GPR30 | Rat left ventricle tissue | 153 | |
| ↓ | ↑ | Not investigated | Rat left ventricle tissue | 157 | |
| NCX | ↑ | ↓ | Not investigated | Rat ventricular tissue | 26 |
| ↑ | Not investigated | Mouse ventricle tissue | 149 | ||
| ↓ | Not investigated | Neonatal rat ventricular cells | 161 | ||
| ↑ | Genomic | Rabbit ventricular cells | 150 | ||
| ─ | ─ | Not investigated | Rat heart tissue | 152 | |
| ↑ | Not investigated | Mouse ventricle tissue | 151 | ||
| ─ | ─ | GPR30 | Rat left ventricle tissue | 153 | |
| ─ | Not investigated | Mouse ventricle tissue | 154 | ||
| Sarcolipin | Not yet documented | ||||
SERCA, sarcoplasmic reticulum Ca2+‐ATPase; NCX, Na+/Ca2+ exchanger pump; ER, oestrogen receptor. ↑ represents upregulation, ↓ represents downregulation and → represents no change.
These studies were carried out in different animal species, disease models, tissue/cell types, age groups, in vivo and ex vivo and using different oestrogen types. Moreover, the observed changes in protein expression due to OVX were reversed by oestrogen replacement.26, 147, 155, 162 This implies that the discrepancies between some of the results could be a result of the experimental variations, and hence, head‐to‐head comparisons might not be possible. Collectively, these findings show that oestrogen status plays a crucial role in the expression and function of cardiac Ca2+‐handling proteins. ERα, ERβ and GPR30 mediate these roles of oestrogen.69, 92, 162 Therefore, the observations that ER and βAR signalling pathways interact may have profound implications on cardiac Ca2+ regulation and contractility. Furthermore, through ERα,7 oestrogen altered myofilament Ca2+ sensitivity.8, 154 Indeed, in a rat model of angiotensin II‐induced hypertension, OVX exacerbated myofilament Ca2+ sensitivity, indicating that oestrogen deficiency may play a role in cardiac disorders by lowering the myofilament sensitivity to Ca2+.8
8. PHARMACOLOGICAL IMPLICATIONS AND THERAPEUTIC OPPORTUNITIES
8.1. Effects of the interactions on drugs targeting βARs and Ca2+ channel blockers
Interactions between ER and βAR pathways could have broad implications in the clinical context. β‐Blockers and Ca2+ channel blockers are 2 mainstays for the treatment of cardiovascular disease.163 These drugs control the heart rate and blood pressure by modulating the activation of βARs. However, there are conflicting observations regarding their effectiveness in managing conditions such as hypertension.163 Reports from cohort studies have noted that some patients, particularly women, under β‐blockers are unable to reach targeted blood pressure compared to men.164 This observation can be explained, partially, by the aforementioned influence of oestrogen on βARs' function. Moreover, gender and age differences in expression of β1ARs/β2ARs have been reported, which may be attributed to oestrogen.135 Besides, gender variations in responses to catecholamines,139 and in cardiac Ca2+ handling,165 have been observed in animal experiments. Therefore, the efficacy of β‐blockers and Ca2+ blockers may vary with gender or age groups based on the interplay between ERs and βARs. As demonstrated, carvedilol, a non‐selective β‐blocker, protected against myocardial contractile dysfunction caused by oestrogen deficiency.158 Interestingly, this is one of the β‐blockers to display biased agonism and it too can activate the β2AR‐Gαi/β‐arrestin pathways.166, 167 With the current understanding of the ER and βAR pathways, further studies should examine how the efficacy of the drugs targeting these receptors and/or their signalling pathways may be altered in the context of the ER and βAR crosstalk. Theoretically, oestrogen by inhibiting the LTCC or altering the expression of βARs might indirectly compromise the functions of Ca2+ blockers and β‐blockers respectively. Consequently, men and women may respond differently to these classes of drugs. The crosstalk may inform the decisions regarding the choice of antihypertensive drugs to patients with consideration to age and gender.
8.2. Therapeutic opportunities
The functional synergism between ERs and βARs provides therapeutic avenues for cardioprotection. For example, while β1AR activation promotes CaMKII‐induced apoptosis,25, 112 β2AR, ERα and GPR30 activation seems to act in a manner that promotes anti‐apoptosis through the Gi/PI3K/Akt pathway. Considerations for strategies targeting the PI3K/Akt pathway will provide a feasible avenue for cardioprotection. For instance, the ability of ERα binding to the alpha subunit of PI3K seems attractive, as it is more specific and avoids various points of integration between ER and βAR signalling cascades discussed above. Activation of Akt pathway will protect against mitochondria‐associated apoptosis induction. Moreover, Akt was shown to act as a surrogate molecular ligand for ERα that induced expression of oestrogen‐regulated cardioprotective genes in breast cancer cells.168 This perspective partly indicates that selective activation of this pathway would potentially enhance the cardiac functions under pathological conditions. Another therapeutic target is the possibility of direct binding of oestrogen to the LTCC. Oestrogen‐LTCC docking studies may help to predict the mode of binding/interaction of this complex. This approach will advance the prospects of oestrogen as a Ca2+ channel blocker if appropriate technologies are applied to enhance its specificity. We anticipate that the therapeutic value of this manoeuvre could be a potential target for Ca2+‐related pathologies such as arrhythmia treatments.
9. CONCLUSION
This review demonstrates the expression patterns and functions of ERs and βARs in cardiovascular tissues. The data sets reviewed above show some inconsistencies with regard to tissue‐specific expression of the ERs. For instance, it is not clear whether ERβ is expressed in adult cardiomyocytes, and the cellular localization of GPR30 is not clear. Therefore, further studies are warranted to resolve these observations. In addition, the reviewed data sets strongly support the hypothesis that ERs and βARs function as collaborative partners in modulating the physiology of the cardiovascular system. The recently described oestrogen receptor GPR30 mimics the dual coupling of the β2ARs to the Gαs and Gαi proteins. On this basis, oestrogen pathways play into the network of the βAR signalling cascades. Furthermore, GPR30 and βARs show similarities with regard to their ability to associate with AKAPs, PDZ motif‐binding proteins and possession of PKA and CaMKII binding sites. Despite the signalling pathways discussed in this review, the functions of the GPR30 and other ERs remain incompletely understood. Further research is required to uncover the identities of signalling molecules that orchestrate their functions.
The crosstalk between the ERs and βARs could have implications on drugs that target these receptors, especially β‐blockers and Ca2+ channel blockers. Oestrogen influences the expression of βARs and Ca2+‐handling proteins, which could compromise the efficacy of the drugs in a gender‐dependent manner. This perspective requires further evaluation at the clinical level. In addition, the concept of direct oestrogen binding to the LTCC might be of great clinical relevance as oestrogen might be used to design Ca2+ channel blockers. This opens a window of research into the receptor‐independent pathways for oestrogen. Furthermore, future research should exploit the therapeutic potential of the cardioprotective PI3K/Akt pathway that is activated downstream of both ERs and βARs.
CONFLICT OF INTEREST
The authors report no conflict of interests.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 81370329), International (Regional) Cooperation and Exchange of NSFC‐RCUK‐MRC (No. 81461138036) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Machuki JO, Zhang HY, Harding SE, Sun H. Molecular pathways of oestrogen receptors and β‐adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol. 2018;222:e12978 https://doi.org/10.1111/apha.12978
REFERENCES
- 1. Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age‐related cardiovascular mortality. PLoS One. 2013;8:e63347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grohé C, Kahlert S, Löbbert K, et al. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107‐112. [DOI] [PubMed] [Google Scholar]
- 3. Pugach EK, Blenck CL, Dragavon JM, Langer SJ, Leinwand LA. Estrogen receptor profiling and activity in cardiac myocytes. Mol Cell Endocrinol. 2016;431:62‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Zhao Z, Lin M, Groban L. Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem. 2015;405:135‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen‐activated protein kinase activity. Arterioscler Thromb Vasc Biol. 2000;20:964‐972. [DOI] [PubMed] [Google Scholar]
- 6. Kuiper GGJM, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors and α and β. Endocrinology. 1997;138:863‐870. [DOI] [PubMed] [Google Scholar]
- 7. Kulpa J, Chinnappareddy N, Pyle WGG. Rapid changes in cardiac myofilament function following the acute activation of estrogen receptor‐alpha. PLoS One. 2012;7:e41076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandit S, Woranush W, Wattanapermpool J, Bupha‐Intr T. Significant role of female sex hormones in cardiac myofilament activation in angiotensin II‐mediated hypertensive rats. J Physiol Sci. 2014;64:269‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercuro G, Podda A, Pitzalis L, et al. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85:787‐789. [DOI] [PubMed] [Google Scholar]
- 10. Debortoli AR, Rouver WDN, Delgado NTB, et al. GPER modulates tone and coronary vascular reactivity in male and female rats. J Mol Endocrinol. 2017;59:171‐180. [DOI] [PubMed] [Google Scholar]
- 11. Valverde LF, Cedillo FD, Ramos ML, Cervera EG, Quijano K, Cordoba J. Changes induced by estradiol‐ethylenediamine derivative on perfusion pressure and coronary resistance in isolated rat heart: L‐type calcium channel. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:27‐32. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H628‐H640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patten RD, Pourati I, Aronovitz MJ, et al. 17 Beta‐estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14:245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giraud GD, Morton MJ, Davis LE, Paul MS, Thornburg KL. Estrogen‐induced left ventricular chamber enlargement in ewes. Am J Physiol. 1993;264(4 Pt 1):E490‐E496. [DOI] [PubMed] [Google Scholar]
- 15. Magness RR, Rosenfeld CR. Local and systemic estradiol‐17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256(4 Pt 1):E536‐E542. [DOI] [PubMed] [Google Scholar]
- 16. Zoma WD, Baker RS, Clark KE. Coronary and uterine vascular responses to raloxifene in the sheep. Am J Obstet Gynecol. 2000;182:521‐528. [DOI] [PubMed] [Google Scholar]
- 17. Zoma WD, Baker RS, Clark KE. Effects of combined use of sildenafil citrate (Viagra) and 17beta‐estradiol on ovine coronary and uterine hemodynamics. Am J Obstet Gynecol. 2004;190:1291‐1297. [DOI] [PubMed] [Google Scholar]
- 18. Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283:H1169‐H1180. [DOI] [PubMed] [Google Scholar]
- 19. Lang U, Baker RS, Clark KE. Estrogen‐induced increases in coronary blood flow are antagonized by inhibitors of nitric oxide synthesis. Eur J Obstet Gynecol Reprod Biol. 1997;74:229‐235. [DOI] [PubMed] [Google Scholar]
- 20. Rosenfeld CR, Jackson GM. Estrogen‐induced refractoriness to the pressor effects of infused angiotensin II. Am J Obstet Gynecol. 1984;148:429‐435. [DOI] [PubMed] [Google Scholar]
- 21. Altan VM, Arioglu E, Guner S, Ozcelikay AT. The influence of diabetes on cardiac beta‐adrenoceptor subtypes. Heart Fail Rev. 2007;12:58‐65. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez‐Arenas A, Aguilar‐Maldonado B, Avendano‐Vazquez SE, Garcia‐Sainz JA. Estrogens cross‐talk to alpha1b‐adrenergic receptors. Mol Pharmacol. 2006;70:154‐162. [DOI] [PubMed] [Google Scholar]
- 23. Walters MR, Sharma R. Cross‐talk between beta‐adrenergic stimulation and estrogen receptors: isoproterenol inhibits 17beta‐estradiol‐induced gene transcription in A7r5 cells. J Cardiovasc Pharmacol. 2003;42:266‐274. [DOI] [PubMed] [Google Scholar]
- 24. Kang S, Liu Y, Sun D, et al. Chronic activation of the G protein‐coupled receptor 30 with agonist G‐1 attenuates heart failure. PLoS One. 2012;7:e48185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Y, Cheng WT, Wu S, Wong TM. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin‐dependent protein kinase II. Br J Pharmacol. 2009;157:705‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium‐handling proteins, β‐adrenergic receptors, and function in rat heart. Life Sci. 2006;79:1257‐1267. [DOI] [PubMed] [Google Scholar]
- 27. Jafri MS. Models of excitation‐contraction coupling in cardiac ventricular myocytes. Methods Mol Biol. 2012;910:309‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G‐protein‐coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607‐617. [DOI] [PubMed] [Google Scholar]
- 29. Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of β‐adrenergic receptor subtypes in blood vessels of knockout mice lacking β1‐ or β2‐adrenergic receptors. Mol Pharmacol. 2001;60:955‐962. [DOI] [PubMed] [Google Scholar]
- 30. Boivin B, Lavoie C, Vaniotis G, et al. Functional β‐adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69‐78. [DOI] [PubMed] [Google Scholar]
- 31. Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hébert TE, Allen BG. Nuclear β‐adrenergic receptors modulate gene expression in adult rat heart. Cell Signal. 2011;23:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boivin B, Chevalier D, Villeneuve LR, Rousseau É, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem. 2003;278:29153‐29163. [DOI] [PubMed] [Google Scholar]
- 33. Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res. 2013;113:739‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall RA, Lefkowitz RJ. Regulation of G protein‐coupled receptor signaling by scaffold proteins. Circ Res. 2002;91:672‐680. [DOI] [PubMed] [Google Scholar]
- 35. Rohrer DK, Desai KH, Jasper JR, et al. Targeted disruption of the mouse beta1‐adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA. 1996;93:7375‐7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evans BA, Sato M, Sarwar M, Hutchinson DS, Summers RJ. Ligand‐directed signalling at β‐adrenoceptors. Br J Pharmacol. 2010;159:1022‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G‐protein‐coupled receptors. Nature. 2009;459:356‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heubach JF, Ravens U, Kaumann AJ. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the gs pathway through human 2‐adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65:1313‐1322. [DOI] [PubMed] [Google Scholar]
- 39. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2‐adrenergic receptor/Gi‐dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126:697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ. Structural basis for receptor subtype‐specific regulation revealed by a chimeric beta 3/beta 2‐adrenergic receptor. Proc Natl Acad Sci USA. 1993;90:3665‐3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gauthier C, Langin D, Balligand J‐L. β3‐adrenoceptors in the cardiovascular system. Trends Pharmacol Sci. 2000;21:426‐431. [DOI] [PubMed] [Google Scholar]
- 42. Hutchinson DS, Bengtsson T, Evans BA, Summers RJ. Mouse beta 3a‐ and beta 3b‐adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol. 2002;135:1903‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hutchinson DS, Sato M, Evans BA, Christopoulos A, Summers RJ. Evidence for pleiotropic signaling at the mouse beta3‐adrenoceptor revealed by SR59230A [3‐(2‐Ethylphenoxy)‐1‐[(1, S)‐1,2,3,4‐tetrahydronapth‐1‐ylamino]‐2S‐2‐propanol oxalate]. J Pharmacol Exp Ther. 2005;312:1064‐1074. [DOI] [PubMed] [Google Scholar]
- 44. Sato M, Hutchinson DS, Bengtsson T, et al. Functional domains of the mouse beta3‐adrenoceptor associated with differential G protein coupling. J Pharmacol Exp Ther. 2005;315:1354‐1361. [DOI] [PubMed] [Google Scholar]
- 45. Sato M, Hutchinson DS, Evans BA, Summers RJ. The beta3‐adrenoceptor agonist 4‐[[(Hexylamino)carbonyl]amino]‐N‐[4‐[2‐[[(2S)‐2‐hydroxy‐3‐(4‐hydroxyphenoxy)prop yl]amino]ethyl]‐phenyl]‐benzenesulfonamide (L755507) and antagonist (S)‐N‐[4‐[2‐[[3‐[3‐(acetamidomethyl)phenoxy]‐2‐hydroxypropyl]amino]‐ethyl]. Mol Pharmacol. 2008;74:1417‐1428. [DOI] [PubMed] [Google Scholar]
- 46. Mizuno K, Kanda Y, Kuroki Y, Nishio M, Watanabe Y. Stimulation of beta(3)‐adrenoceptors causes phosphorylation of p38 mitogen‐activated protein kinase via a stimulatory G protein‐dependent pathway in 3T3‐L1 adipocytes. Br J Pharmacol. 2002;135:951‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skeberdis VA, Gendviliene V, Zablockaite D, et al. β3‐adrenergic receptor activation increases human atrial tissue contractility and stimulates the L‐type Ca2+ current. J Clin Invest. 2008;118:3219‐3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Treinys R, Zablockaite D, Gendviliene V, Jurevičius J, Skeberdis VA. β3‐adrenergic regulation of l‐type Ca2+ current and force of contraction in human ventricle. J Membr Biol. 2014;247:309‐318. [DOI] [PubMed] [Google Scholar]
- 49. Sterin‐Borda L, Bernabeo G, Ganzinelli S, Joensen L, Borda E. Role of nitric oxide/cyclic GMP and cyclic AMP in β3 adrenoceptor‐chronotropic response. J Mol Cell Cardiol. 2006;40:580‐588. [DOI] [PubMed] [Google Scholar]
- 50. Vaniotis G, Glazkova I, Merlen C, et al. Regulation of cardiac nitric oxide signaling by nuclear β‐adrenergic and endothelin receptors. J Mol Cell Cardiol. 2013;62:58‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richalet J‐P. Physiological and clinical implications of adrenergic pathways at high altitude. Adv Exp Med Biol. 2016;903:343‐356. [DOI] [PubMed] [Google Scholar]
- 52. Weiss S, Oz S, Benmocha A, Dascal N. Regulation of cardiac L‐Type Ca2+ channel CaV1.2 via the β‐adrenergic‐cAMP‐protein kinase a pathway: old dogmas, advances, and new uncertainties. Circ Res. 2013;113:617‐631. [DOI] [PubMed] [Google Scholar]
- 53. Ulucan C, Wang X, Baljinnyam E, et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662‐H1672. [DOI] [PubMed] [Google Scholar]
- 54. Lezoualc'H F, Fazal L, Laudette M, Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ Res. 2016;118:881‐897. [DOI] [PubMed] [Google Scholar]
- 55. Zhu W, Tsang S, Browe DM, et al. Interaction of β1‐adrenoceptor with RAGE mediates cardiomyopathy via CaMKII signaling. JCI Insight. 2016;1:e84969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Erickson JR. Mechanisms of CaMKII activation in the heart. Front Pharmacol. 2014;5:59 APR. https://doi.org/10.3389/fphar.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dries E, Santiago DJ, Johnson DM, et al. Calcium/calmodulin‐dependent kinase II and nitric oxide synthase 1‐dependent modulation of ryanodine receptors during β‐adrenergic stimulation is restricted to the dyadic cleft. J Physiol. 2016;594:5923‐5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tonegawa K, Otsuka W, Kumagai S, et al. Caveolae‐specific activation loop between CaMKII and L‐type Ca2+ channel aggravates cardiac hypertrophy in α1‐adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2017;312:H501‐H514. [DOI] [PubMed] [Google Scholar]
- 59. Feng N, Anderson ME. CaMKII is a nodal signal for multiple programmed cell death pathways in heart. J Mol Cell Cardiol. 2017;103:102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23:75‐86. [DOI] [PubMed] [Google Scholar]
- 61. Nuedling S, Kahlert S, Loebbert K, et al. 17β‐estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in‐vitro and in‐vivo. Cardiovasc Res. 1999;43:666‐674. [DOI] [PubMed] [Google Scholar]
- 62. Kabir ME, Singh H, Lu R, Olde B, Leeb‐Lundberg LMF, Bopassa JC. G protein‐coupled estrogen receptor 1 mediates acute estrogen‐induced cardioprotection via MEK/ERK/GSK‐3β Pathway after Ischemia/Reperfusion. PLoS One. 2015;10:e0135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625‐1630. [DOI] [PubMed] [Google Scholar]
- 64. Broselid S, Berg KA, Chavera TA, et al. G protein‐coupled receptor 30 (GPR30) forms a plasma membrane complex with membrane‐associated guanylate kinases (MAGUKs) and protein kinase a‐anchoring protein 5 (AKAP5) that constitutively inhibits cAMP production. J Biol Chem. 2014;289:22117‐22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender‐specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem. 2010;26:457‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mahmoodzadeh S, Eder S, Nordmeyer J, et al. Estrogen receptor alpha up‐regulation and redistribution in human heart failure. FASEB J. 2006;20:926‐934. [DOI] [PubMed] [Google Scholar]
- 67. Nordmeyer J, Eder S, Mahmoodzadeh S, et al. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation. 2004;110:3270‐3275. [DOI] [PubMed] [Google Scholar]
- 68. Grohé C, Kahlert S, Löbbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol. 1998;156:R1‐R7. [DOI] [PubMed] [Google Scholar]
- 69. Yang X, Chen G, Papp R, Defranco DB, Zeng F, Salama G. Oestrogen upregulates L‐type Ca2+ channels via oestrogen‐receptor‐ by a regional genomic mechanism in female rabbit hearts. J Physiol. 2012;590:493‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ricchiuti V, Lian CG, Oestreicher EM, et al. Estradiol increases angiotensin II type 1 receptor in hearts of ovariectomized rats. J Endocrinol. 2009;200:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905‐931. [DOI] [PubMed] [Google Scholar]
- 72. O'Dowd BF, Nguyen T, Marchese A, et al. Discovery of three novel G‐protein‐coupled receptor genes. Genomics. 1998;47:310‐313. [DOI] [PubMed] [Google Scholar]
- 73. Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P. Plant‐derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J Am Coll Cardiol. 2000;35:1977‐1985. [DOI] [PubMed] [Google Scholar]
- 74. Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403‐409. [DOI] [PubMed] [Google Scholar]
- 75. Koganti S, Snyder R, Gumaste U, Karamyan VT, Thekkumkara T. 2‐Methoxyestradiol binding of GPR30 down‐regulates angiotensin AT 1 receptor. Eur J Pharmacol. 2014;723:131‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Evans NJ, Bayliss AL, Reale V, Evans PD. Characterisation of signalling by the Endogenous GPER1 (GPR30) Receptor in an Embryonic Mouse Hippocampal Cell Line (mHippoE‐18). PLoS One. 2016;11:e0152138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lin AHY, Li RWS, Ho EYW, et al. Differential Ligand Binding Affinities of Human Estrogen Receptor‐α Isoforms. PLoS One. 2013;8:e63199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bao TZ, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure‐activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132‐4150. [DOI] [PubMed] [Google Scholar]
- 79. Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76:414‐418. [DOI] [PubMed] [Google Scholar]
- 80. Cornil CA, Ball GF, Balthazart J. The dual action of estrogen hypothesis. Trends Neurosci. 2015;38:408‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim S‐C, Seo K‐K, Myung S‐C, Lee MY. Relaxation of rabbit cavernous smooth muscle to 17beta‐estradiol: a non‐genomic. NO‐independent mechanism. Asian J Androl. 2004;6:127‐131. [PubMed] [Google Scholar]
- 82. Chambliss KL, Yuhanna IS, Anderson RGW, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938‐946. [DOI] [PubMed] [Google Scholar]
- 83. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704‐19710. [DOI] [PubMed] [Google Scholar]
- 84. Le HH, Belcher SM. Rapid signaling actions of environmental estrogens in developing granule cell neurons are mediated by estrogen receptors. Endocrinology. 2010;151:5689‐5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. Cross‐talk between membrane‐initiated and nuclear‐initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ullrich ND, Koschak A, MacLeod KT. Oestrogen directly inhibits the cardiovascular L‐type Ca2+ channel Cav1.2. Biochem Biophys Res Commun. 2007;361:522‐527. [DOI] [PubMed] [Google Scholar]
- 87. Rybalchenko V, Grillo MA, Gastinger MJ, Rybalchenko N, Payne AJ, Koulen P. The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+ . J Recept Signal Transduct Res. 2009;29:326‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERα) and beta (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277‐4291. [DOI] [PubMed] [Google Scholar]
- 89. Kow L‐M, Pfaff DW. Rapid estrogen actions on ion channels: a survey in search for mechanisms. Steroids. 2016;111:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bolego C, Cignarella A, Sanvito P, et al. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor‐alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203‐1208. [DOI] [PubMed] [Google Scholar]
- 91. Wu Q, Zhao Z, Sun H, Hao Y, Yan C, Gu S. Oestrogen changed cardiomyocyte contraction and beta‐adrenoceptor expression in rat hearts subjected to ischaemia‐reperfusion. Exp Physiol. 2008;93:1034‐1043. [DOI] [PubMed] [Google Scholar]
- 92. Schuster I, Mahmoodzadeh S, Dworatzek E, et al. Cardiomyocyte‐specific overexpression of oestrogen receptor improves survival and cardiac function after myocardial infarction in female and male mice. Clin Sci. 2016;130:365‐376. [DOI] [PubMed] [Google Scholar]
- 93. Hill BJF, Muldrew E. Estrogen upregulates the sarcoplasmic reticulum Ca2+‐ATPase pump in coronary arteries. Clin Exp Pharmacol Physiol. 2014;41:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Johnson BD, Zheng W, Korach KS, Scheuer T, Catterall WA, Rubanyi GM. Increased expression of the cardiac L‐type calcium channel in estrogen receptor‐deficient mice. J Gen Physiol. 1997;110:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein‐coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737‐741. [DOI] [PubMed] [Google Scholar]
- 96. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624‐632. [DOI] [PubMed] [Google Scholar]
- 97. Bhattacharya S, Hall SE, Li H, Vaidehi N. Ligand‐stabilized conformational states of human beta(2) adrenergic receptor: insight into G‐protein‐coupled receptor activation. Biophys J. 2008;94:2027‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang T, McDonald C, Petrenko NB, et al. Estrogen‐related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35:1281‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tran QK, VerMeer M. Biosensor‐based approach identifies four distinct calmodulin‐binding domains in the G protein‐coupled estrogen receptor 1. PLoS One. 2014;9:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Filardo EJ, Quinn JA, Frackelton ARJ, Bland KI. Estrogen action via the G protein‐coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP‐mediated attenuation of the epidermal growth factor receptor‐to‐MAPK signaling axis. Mol Endocrinol. 2002;16:70‐84. [DOI] [PubMed] [Google Scholar]
- 101. Yu X. The Non‐genomic Signaling Pathways Mediated By G‐protein Coupled Estrogen Receptor 1 (GPER) In Coronary Arteries. Dr Diss Texas A M Univ 2014.
- 102. Ding Q, Gros R, Limbird LE, Chorazyczewski J, Feldman RD. Estradiol‐mediated ERK phosphorylation and apoptosis in vascular smooth muscle cells requires GPR 30. Am J Physiol Cell Physiol. 2009;297:C1178‐C1187. [DOI] [PubMed] [Google Scholar]
- 103. Zucchetti AE, Barosso IR, Boaglio AC, et al. G‐protein‐coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17ß‐d‐glucuronide‐induced cholestasis. Hepatology. 2014;59:1016‐1029. [DOI] [PubMed] [Google Scholar]
- 104. Fu Q, Chen X, Xiang YK. Compartmentalization of β‐adrenergic signals in cardiomyocytes. Trends Cardiovasc Med. 2013;23:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shen JX, Cooper DMF. AKAP79, PKC, PKA and PDE4 participate in a Gq‐linked muscarinic receptor and adenylate cyclase 2 cAMP signalling complex. Biochem J. 2013;455:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002;277:33783‐33790. [DOI] [PubMed] [Google Scholar]
- 107. Akama KT, Thompson LI, Milner TA, McEwen BS. Post‐synaptic density‐95 (PSD‐95) binding capacity of G‐protein‐coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288:6438‐6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xiang K. The PDZ‐binding motif of the beta2‐adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc Natl Acad Sci USA. 2003;100:10776‐10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li X, Nooh MM, Bahouth SW. Role of AKAP79/150 protein in β1‐adrenergic receptor trafficking and signaling in mammalian cells. J Biol Chem. 2013;288:33797‐33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79‐mediated targeting of the cyclic AMP‐dependent protein kinase to the β1‐adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281:33537‐33553. [DOI] [PubMed] [Google Scholar]
- 111. Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97‐AKAP79‐cAMP‐dependent protein kinase scaffold at the type 1 PSD‐95/DLG/ZO1 motif of the human β1‐adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282:5085‐5099. [DOI] [PubMed] [Google Scholar]
- 112. Yoo B, Lemaire A, Mangmool S, et al. Beta1‐adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Hear Circ Physiol. 2009;297:H1377‐H1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tran QK, Firkins R, Giles J, et al. Estrogen enhances linkage in the vascular endothelial calmodulin network via a feedforward mechanism at the G protein‐coupled estrogen receptor 1. J Biol Chem. 2016;291:10805‐10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cao X, Zhou C, Chong J, et al. Estrogen resisted stress‐induced cardiomyopathy through increasing the activity of β2AR‐Gαs signal pathway in female rats. Int J Cardiol. 2015;187:377‐386. [DOI] [PubMed] [Google Scholar]
- 115. Patten RD, Pourati I, Aronovitz MJ, et al. 17β‐estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho‐inositide‐3 kinase/Akt signaling. Circ Res. 2004;95:692‐699. [DOI] [PubMed] [Google Scholar]
- 116. Li W‐L, Xiang W, Ping Y. Activation of novel estrogen receptor GPER results in inhibition of cardiocyte apoptosis and cardioprotection. Mol Med Rep. 2015;12:2425‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Xu C, Liu A, Sun H, et al. β2 ‐Adrenoceptor confers cardioprotection against hypoxia in isolated ventricular myocytes and the effects depend on estrogenic environment. J Recept Signal Transduct. 2010;30:255‐261. [DOI] [PubMed] [Google Scholar]
- 118. Chesley A, Lundberg MS, Asai T, et al. The β2‐adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi‐dependent coupling to phosphatidylinositol 3′‐kinase. Circ Res. 2000;87:1172‐1179. [DOI] [PubMed] [Google Scholar]
- 119. Simoncini T, Hafezi‐Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol‐3‐OH kinase. Nature. 2000;407:538‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fraser IDC, Cong M, Kim J, et al. Assembly of an A kinase‐anchoring protein‐β2‐adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409‐412. [DOI] [PubMed] [Google Scholar]
- 121. Balligand J‐L. Cardiac salvage by tweaking with β3‐adrenergic receptors. Cardiovasc Res. 2016;111:128‐133. [DOI] [PubMed] [Google Scholar]
- 122. Germack R, Dickenson JM. Induction of beta3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J Pharmacol Exp Ther. 2006;316:392‐402. [DOI] [PubMed] [Google Scholar]
- 123. Trappanese DM, Liu Y, McCormick RC, et al. Chronic beta1‐adrenergic blockade enhances myocardial beta3‐adrenergic coupling with nitric oxide‐cGMP signaling in a canine model of chronic volume overload: new insight into mechanisms of cardiac benefit with selective beta1‐blocker therapy. Basic Res Cardiol. 2015;110:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chruscinski AJ, Singh H, Chan SM, Utz PJ. Broad‐scale phosphoprotein profiling of beta adrenergic receptor (β‐AR) signaling reveals novel Phosphorylation and Dephosphorylation events. PLoS One. 2013;8:e82164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bhatnagar A, Sheffler DJ, Kroeze WK, Compton‐Toth B, Roth BL. Caveolin‐1 interacts with 5‐HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq‐coupled protein receptors. J Biol Chem. 2004;279:34614‐34623. [DOI] [PubMed] [Google Scholar]
- 126. Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta ‐adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447‐41457. [DOI] [PubMed] [Google Scholar]
- 127. Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far‐reaching beta1‐adrenergic but locally confined beta2‐adrenergic receptor‐mediated signaling. Circ Res. 2006;99:1084‐1091. [DOI] [PubMed] [Google Scholar]
- 128. Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L‐type Ca2+ channels to a caveolar macromolecular signaling complex is required for beta(2)‐adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:7500‐7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Timofeyev V, Myers RE, Kim HJ, et al. Adenylyl cyclase subtype‐specific compartmentalization: differential regulation of L‐type Ca2+ current in ventricular myocytes. Circ Res. 2013;112:1567‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chambliss KL, Yuhanna IS, Mineo C, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44‐E52. [DOI] [PubMed] [Google Scholar]
- 131. Ke X, Shu X‐R, Wu F, et al. Overexpression of the beta2AR gene improves function and re‐endothelialization capacity of EPCs after arterial injury in nude mice. Stem Cell Res Ther. 2016;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG, Lisanti MP. Caveolin‐1 potentiates estrogen receptor alpha (ERalpha) signaling. caveolin‐1 drives ligand‐independent nuclear translocation and activation of ERalpha. J Biol Chem. 1999;274:33551‐33556. [DOI] [PubMed] [Google Scholar]
- 133. Sato M, Hutchinson DS, Halls ML, et al. Interaction with caveolin‐1 modulates G protein coupling of mouse beta3‐adrenoceptor. J Biol Chem. 2012;287:20674‐20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kumar P, Wu Q, Chambliss KL, et al. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370‐1380. [DOI] [PubMed] [Google Scholar]
- 135. Lindenfeld J, Cleveland JC, Kao DP, et al. Sex‐related differences in age‐associated downregulation of human ventricular myocardial β1‐adrenergic receptors. J Hear Lung Transplant. 2016;35:352‐361. [DOI] [PubMed] [Google Scholar]
- 136. Lohse MJ, Engelhardt S, Eschenhagen T. What Is the Role of β‐Adrenergic Signaling in Heart Failure? Circ Res. 2003;93:896‐906. [DOI] [PubMed] [Google Scholar]
- 137. Kam KWL, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β1‐adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004;309:8‐15. [DOI] [PubMed] [Google Scholar]
- 138. Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of β1‐adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813‐1824. [DOI] [PubMed] [Google Scholar]
- 139. Kocic I, Gruchala M, Petrusewicz J. Pretreatment of male guinea pigs by 17β‐estradiol induces hypersensitivity of β‐adrenoceptors in electrically driven left atria. Int J Cardiol. 2008;129:22‐25. [DOI] [PubMed] [Google Scholar]
- 140. Liu A, Gao L, Kang S, et al. Testosterone enhances estradiol's cardioprotection in ovariectomized rats. J Endocrinol. 2012;212:61‐69. [DOI] [PubMed] [Google Scholar]
- 141. Dent MR, Tappia PS, Dhalla NS. Gender related alterations of β‐adrenoceptor mechanisms in heart failure due to arteriovenous fistula. J Cell Physiol. 2012;227:3080‐3087. [DOI] [PubMed] [Google Scholar]
- 142. Monjo M, Pujol E, Roca P. α2‐ to β3‐Adrenoceptor switch in 3T3‐L1 preadipocytes and adipocytes: modulation by testosterone, 17β‐estradiol, and progesterone. Am J Physiol Endocrinol Metab. 2005;289:E145‐E150. [DOI] [PubMed] [Google Scholar]
- 143. Malo A, Puerta M. Oestradiol and progesterone change β3‐adrenergic receptor affinity and density in brown adipocytes. Eur J Endocrinol. 2001;145:87‐91. [DOI] [PubMed] [Google Scholar]
- 144. Willis BC, Salazar‐Cantú A, Silva‐Platas C, et al. Impaired oxidative metabolism and calcium mishandling underlie cardiac dysfunction in a rat model of post‐acute isoproterenol‐induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308:H467‐H477. [DOI] [PubMed] [Google Scholar]
- 145. Shanmugam M, Li D, Gao S, et al. Cardiac specific expression of threonine 5 to alanine mutant sarcolipin results in structural remodeling and diastolic dysfunction. PLoS One. 2015;10:e0115822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kusch A, Schmidt M, Gürgen D, et al. 17β‐Estradiol regulates mTORC2 sensitivity to rapamycin in adaptive cardiac remodeling. PLoS One. 2015;10:33797‐33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bupha‐Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2006;291:H1101‐H1108. [DOI] [PubMed] [Google Scholar]
- 148. Liu C‐G, Xu K‐Q, Xu X, et al. 17β‐oestradiol regulates the expression of Na+/K+‐ATPase β1‐subunit, sarcoplasmic reticulum Ca2+‐ATPase and carbonic anhydrase iv in H9C2 cells. Clin Exp Pharmacol Physiol. 2007;34:998‐1004. [DOI] [PubMed] [Google Scholar]
- 149. Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133:174‐185. [DOI] [PubMed] [Google Scholar]
- 150. Chen G, Yang X, Alber S, Shusterman V, Salama G. Regional genomic regulation of cardiac sodium‐calcium exchanger by oestrogen. J Physiol. 2011;589:1061‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Haddad R, Kasneci A, Sebag IA, Chalifour LE. Cardiac structure/function, protein expression, and DNA methylation are changed in adult female mice exposed to diethylstilbestrol in utero. Can J Physiol Pharmacol. 2013;91:741‐749. [DOI] [PubMed] [Google Scholar]
- 152. Kravtsov GM, Kam KWL, Liu J, Wu S, Wong TM. Altered Ca2+ handling by ryanodine receptor and Na+‐Ca2+ exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol. 2007;292:C1625‐C1635. [DOI] [PubMed] [Google Scholar]
- 153. Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res. 2012;94:96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fares E, Pyle WG, Ray G, et al. The impact of ovariectomy on calcium homeostasis and myofilament calcium sensitivity in the aging mouse heart. PLoS One. 2013;8:e74719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ribeiro RF Jr, Pavan BM, Potratz FF, et al. Myocardial contractile dysfunction induced by ovariectomy requires AT(1)Receptor activation in female rats. Cell Physiol Biochem. 2012;30:1‐12. [DOI] [PubMed] [Google Scholar]
- 156. Dunay GA, Paragi P, Sára L, et al. Depressed calcium cycling contributes to lower ischemia tolerance in hearts of estrogen‐deficient rats. Menopause. 2015;22:773‐782. [DOI] [PubMed] [Google Scholar]
- 157. Paigel AS, Ribeiro RF, Fernandes AA, Targueta GP, Vassallo DV, Stefanon I. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod Biol Endocrinol. 2011;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ribeiro RF, Potratz FF, Pavan BMM, et al. Carvedilol prevents ovariectomy‐induced myocardial contractile dysfunction in female rat. PLoS One. 2013;8:e53226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ren J, Hintz KK, Roughead ZKF, et al. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284:H1800‐H1807. [DOI] [PubMed] [Google Scholar]
- 160. Bell JR, Bernasochi GB, Varma U, et al. Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice. Am J Physiol Heart Circ Physiol. 2014;306:H1265‐H1274. [DOI] [PubMed] [Google Scholar]
- 161. Mu X, Harvey P. Estrogen differentially affects expression of calcium handling genes in female and male adult cardiomyocytes. J Stud Res. 2012;1:31‐37. [Google Scholar]
- 162. Alencar AK, da Silva JS, Lin M, et al. Effect of age, estrogen status, and late‐life GPER activation on cardiac structure and function in the Fischer344x Brown Norway female rat. J Gerontol A Biol Sci Med Sci. 2017;72:152‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chatterjee S, Biondi‐Zoccai G, Abbate A, et al. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta‐analysis. BMJ. 2013;23:26‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ljungman C, Kahan T, Schioler L, et al. Gender differences in antihypertensive drug treatment: results from the Swedish Primary Care Cardiovascular Database (SPCCD). J Am Soc Hypertens. 2014;8:882‐890. [DOI] [PubMed] [Google Scholar]
- 165. Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E. Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol. Am J Physiol Heart Circ Physiol. 2003;285:H2657‐H2662. [DOI] [PubMed] [Google Scholar]
- 166. Gong H, Sun H, Koch WJ, et al. Specific beta(2)AR blocker ICI 118,551 actively decreases contraction through a G(i)‐coupled form of the beta(2)AR in myocytes from failing human heart. Circulation. 2002;105:2497‐2503. [DOI] [PubMed] [Google Scholar]
- 167. Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of beta‐blockers at the human beta 2‐adrenoceptor provide evidence for agonist‐directed signaling. Mol Pharmacol. 2003;64:1357‐1369. [DOI] [PubMed] [Google Scholar]
- 168. Campbell RA, Bhat‐Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3‐kinase/AKT‐mediated activation of estrogen receptor α: a new model for anti‐estrogen resistance. J Biol Chem. 2001;276:9817‐9824. [DOI] [PubMed] [Google Scholar]
- 169. Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996‐2009. [DOI] [PubMed] [Google Scholar]
- 170. Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA. 2003;100:4807‐4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Simoncini T, Rabkin E, Liao JK. Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3‐kinase in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:198‐203. [DOI] [PubMed] [Google Scholar]
- 172. Chen Z, Yuhanna IS, Galcheva‐Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Liu M‐M, Albanese C, Anderson CM, et al. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353‐24360. [DOI] [PubMed] [Google Scholar]
- 174. Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson J‐A. Estrogen receptor (ER) beta modulates ERalpha‐mediated transcriptional activation by altering the recruitment of c‐Fos and c‐Jun to estrogen‐responsive promoters. Mol Endocrinol. 2006;20:534‐543. [DOI] [PubMed] [Google Scholar]
- 175. Gao X, Liang Q, Chen Y, Wang H‐S. Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology. 2013;154:4607‐4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor‐alpha36, a novel variant of human estrogen receptor‐alpha66. Biochem Biophys Res Commun. 2005;336:1023‐1027. [DOI] [PubMed] [Google Scholar]
- 177. Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v‐erb‐A. Nature. 1986;320:134‐139. [DOI] [PubMed] [Google Scholar]
- 178. Enmark E, Pelto‐Huikko M, Grandien K, et al. Human estrogen receptor beta‐gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258‐4265. [DOI] [PubMed] [Google Scholar]
- 179. Lu B, Leygue E, Dotzlaw H, Murphy LJ, Murphy LC, Watson PH. Estrogen receptor‐beta mRNA variants in human and murine tissues. Mol Cell Endocrinol. 1998;138:199‐203. [DOI] [PubMed] [Google Scholar]
- 180. Holm A, Hellstrand P, Olde B, Svensson D, Leeb‐Lundberg LMF, Nilsson B‐O. The G protein‐coupled estrogen receptor 1 (GPER1/GPR30) agonist G‐1 regulates vascular smooth muscle cell Ca2+ handling. J Vasc Res. 2013;50:421‐429. [DOI] [PubMed] [Google Scholar]
- 181. Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207‐212. [DOI] [PubMed] [Google Scholar]
- 182. Little AG, Seebacher F. Temperature determines toxicity: bisphenol A reduces thermal tolerance in fish. Environ Pollut. 2015;197:84‐89. [DOI] [PubMed] [Google Scholar]
- 183. Turdi S, Huff AF, Pang J, et al. 17β‐estradiol attenuates ovariectomy‐induced changes in cardiomyocyte contractile function via activation of AMP‐activated protein kinase. Toxicol Lett. 2015;232:253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]


