Figure EV3. Evi is ubiquitinated and degraded from the ER by the proteasome.
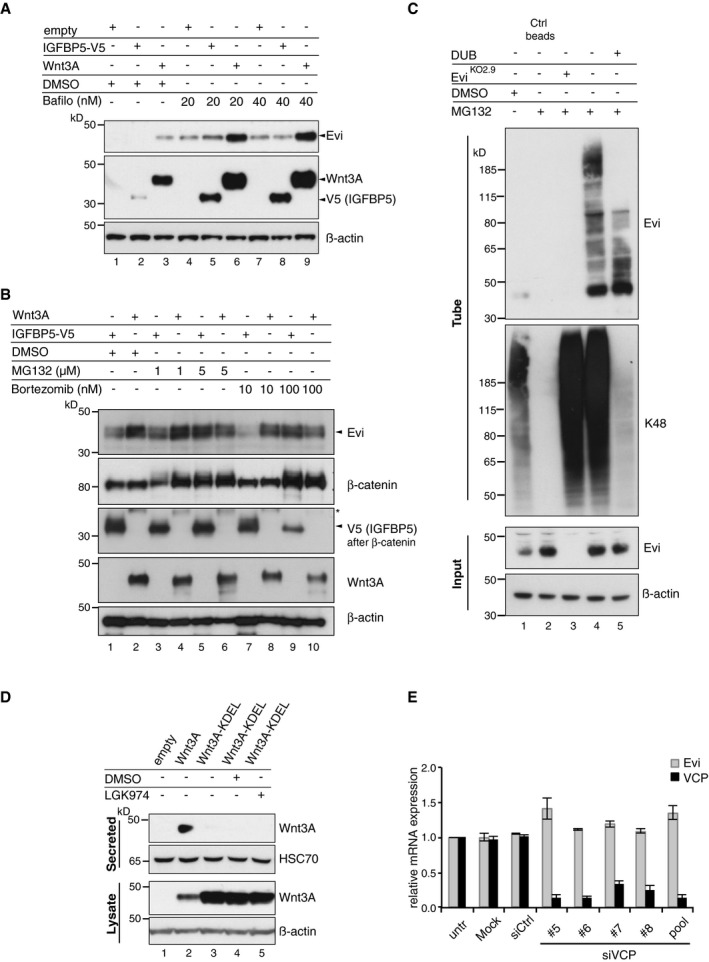
- HEK293T cells were transfected with the indicated expression constructs and treated with Bafilomycin A (Bafilo) in the indicated concentrations for 24 h. Lysosomal inhibition additionally increased Evi protein on top of Wnt3A expression indicating that Evi is degraded by the lysosome also in the presence of Wnt proteins.
- HEK293T cells were transfected with Wnt3A or IGFBP5‐V5 expression plasmids and treated for 24 h with DMSO, MG132, or bortezomib in the indicated concentrations.
- Following MG132 treatment (1 μM for 24 h), poly‐ubiquitinated proteins were precipitated using TUBE2 agarose and incubated with DUB buffer, supplemented with the catalytic domain of the DUB enzyme USP2, if indicated. The precipitates were assayed for endogenous Evi or K48 poly‐ubiquitin. Ctrl agarose beads were used to confirm specificity of the TUBE2 assay for ubiquitinated proteins.
- HEK293T cells were transfected with the indicated overexpression constructs and treated with 5 μM LGK974 for 48 h, if indicated. Secreted Wnt3A proteins were precipitated from conditioned medium and analyzed via immunoblotting. Compared to wild‐type Wnt3A, Wnt3A‐KDEL is not secreted into the medium affirming cellular retention. HSC70 served as loading control. All Western blots are representative of three independent experiments.
- Upon VCP knockdown in HEK293T cells using single or pooled siRNAs, Evi and VCP mRNA were analyzed via qRT–PCR. mRNA levels are shown as mean ± s.d. relative to GAPDH mRNA levels from three independent experiments.
Source data are available online for this figure.
