The adapter protein 4 is involved in plant immunity associated with vacuolar-plasma membrane fusion and in hypersensitive cell death triggered by type-III effector recognition on the plasma membrane.
Abstract
Plant immunity to avirulent bacterial pathogens is associated with subcellular membrane dynamics including fusion between the vacuolar and plasma membranes, resulting in hypersensitive cell death. Here, we report that ADAPTOR PROTEIN COMPLEX-4 (AP-4) subunits are involved in plant immunity associated with hypersensitive cell death. We isolated a mutant with a defect in resistance to an avirulent strain of Pseudomonas syringae pv. tomato (Pto) DC3000 avrRpm1 from a vacuolar protein sorting mutant library of Arabidopsis (Arabidopsis thaliana). The mutant was identical to gfs4-1, which has a mutation in the gene encoding the AP-4 subunit AP4B. Thus, we focused on AP4B and another subunit, AP4E. All of the mutants (ap4b-3, ap4b-4, ap4e-1, and ap4e-2) were defective in hypersensitive cell death and resistance to Pto DC3000 with the type III effector AvrRpm1 or AvrRpt2, both of which are recognized on the plasma membrane, while they showed slightly enhanced susceptibility to the type-III-secretion-deficient P. syringae strain hrcC. On the other hand, both ap4b-3 and ap4b-4 showed no defect in resistance to Pto DC3000 with the type III effector AvrRps4, which is recognized in the cytosol and does not induce hypersensitive cell death. Upon infection with Pto DC3000 avrRpt2, the ap4b-3 and ap4b-4 leaf cells did not show fusion between vacuolar and plasma membranes, whereas the wild-type leaf cells did. These results suggest that AP-4 contributes to cell death-associated immunity, possibly via membrane fusion, after type III effector-recognition on the plasma membrane.
Pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) are two modes of plant immunity (for review, see Jones and Dangl, 2006; Tsuda and Katagiri, 2010). PTI is triggered by the recognition of microbe/pathogen-associated molecular patterns by pattern-recognition receptors. PTI acts as a basal defense response that protects plants from invasion by most potentially pathogenic microbes. ETI is induced by the specific recognition of pathogen effectors by disease resistance (R) proteins (Yang et al., 1997; Dangl and Jones, 2001). ETI is often accompanied by the hypersensitive response (HR), which is characterized by rapid and localized programmed cell death (PCD), a process referred to as hypersensitive cell death (Coll et al., 2011; Hara-Nishimura and Hatsugai, 2011).
Vacuoles play important roles in plant immunity (for review, see Iglesias and Meins, 2000; Hatsugai and Hara-Nishimura, 2010). The vacuole, which typically occupies most of the plant cell volume, contains immune-related proteins that are used against invading pathogens, as well as hydrolases and lipases that degrade cellular materials that are no longer required. Plant cells infected with avirulent bacterial pathogens discharge their vacuolar contents into the extracellular space via fusion of the vacuolar membrane to the plasma membrane, leading to the suppression of extracellular bacterial growth and hypersensitive cell death (Hatsugai et al., 2009). On the other hand, vacuolar membrane collapse is observed during infection by avirulent viruses and fungi. This process releases the vacuolar contents into the cytoplasm and leads to hypersensitive cell death as well, thereby preventing pathogen growth (Hatsugai et al., 2004, 2015; Kuroyanagi et al., 2005; Higaki et al., 2007).
Soluble vacuolar proteins, such as seed storage proteins and lytic enzymes, are synthesized on the rough endoplasmic reticulum and transported into protein storage vacuoles and lytic vacuoles, respectively, via the endomembrane system. The proteins often contain vacuolar sorting signals that can be recognized by VACUOLAR SORTING RECEPTOR 1 (VSR1) in the trans-Golgi network (TGN; Shimada et al., 2003; Fuji et al., 2007; Zouhar et al., 2010). To sort the receptors in the TGN into vacuoles, the adaptor protein (AP) complex binds to the cytosolic domains of the receptors (Robinson, 2004). We recently reported that the AP-4 complex, which is localized to the TGN subdomain, functions in VSR1-mediated vacuolar protein sorting in Arabidopsis (Arabidopsis thaliana; Fuji et al., 2016). AP-4 is a heterotetrameric complex consisting of two large subunits (β4 and ε), one medium subunit (μ4), and one small subunit (σ4) (Robinson, 2004).
We previously reported that a Beige and Chediak-Higashi domain protein is involved in vacuolar protein transport and is required for full-strength ETI in Arabidopsis (Teh et al., 2015). Arabidopsis lazarus 4 (laz4), a suppressor mutant of accelerated cell death11 (acd11), partly suppresses immunity- and PCD-related phenotypes in acd11 (Munch et al., 2015). LAZ4 encodes VACUOLAR PROTEIN SORTING 35B, a component of the multisubunit retromer complex required for vacuolar transport of storage proteins to protein storage vacuoles in seeds (Yamazaki et al., 2008; Munch et al., 2015). The presence of laz4 suppresses acd11 phenotypes, indicating that vacuolar protein transport is involved in plant immunity. However, how vacuolar protein transport contributes to plant immunity is not yet fully understood.
We previously developed a high-throughput screening system to isolate vacuolar sorting-deficient mutants in Arabidopsis, designated green fluorescent seed (gfs) mutants, using GFP as a reporter (Fuji et al., 2007). Arabidopsis seeds harboring GFP-CT24, encoding a signal peptide and GFP followed by the 24 C-terminal amino acids of β-conglycinin (CT24) under the control of a seed-specific promoter, accumulate this GFP fusion protein in their protein storage vacuoles (Nishizawa et al., 2003). By contrast, gfs mutant seeds mis-sort GFP-CT24 by secreting it from the cell, resulting in seeds exhibiting strong green fluorescence (Fuji et al., 2007). Plants likely use common systems for vacuolar protein transport in seeds and vegetative organs, because Arabidopsis mutants with a defect in vacuolar protein transport in seeds exhibit a defect in vacuolar protein transport in vegetative organs (Shimada et al., 2006; Yamazaki et al., 2008). Thus, the gfs mutants likely have vacuolar protein transport defects in vegetative organs.
In this study, we show that AP-4 complex is involved in plant immunity associated with fusion between the vacuolar membrane and the plasma membrane. The deficiency in AP-4 components abolished membrane fusion and hypersensitive cell death upon infection with an avirulent bacterial strain and reduced resistance to this strain. Our results provide valuable insights into the role of AP-4-dependent vacuolar protein sorting in ETI.
RESULTS
Isolation of a Vacuolar Protein Sorting Mutant That Also Has a Defect in Resistance to Avirulent Bacteria
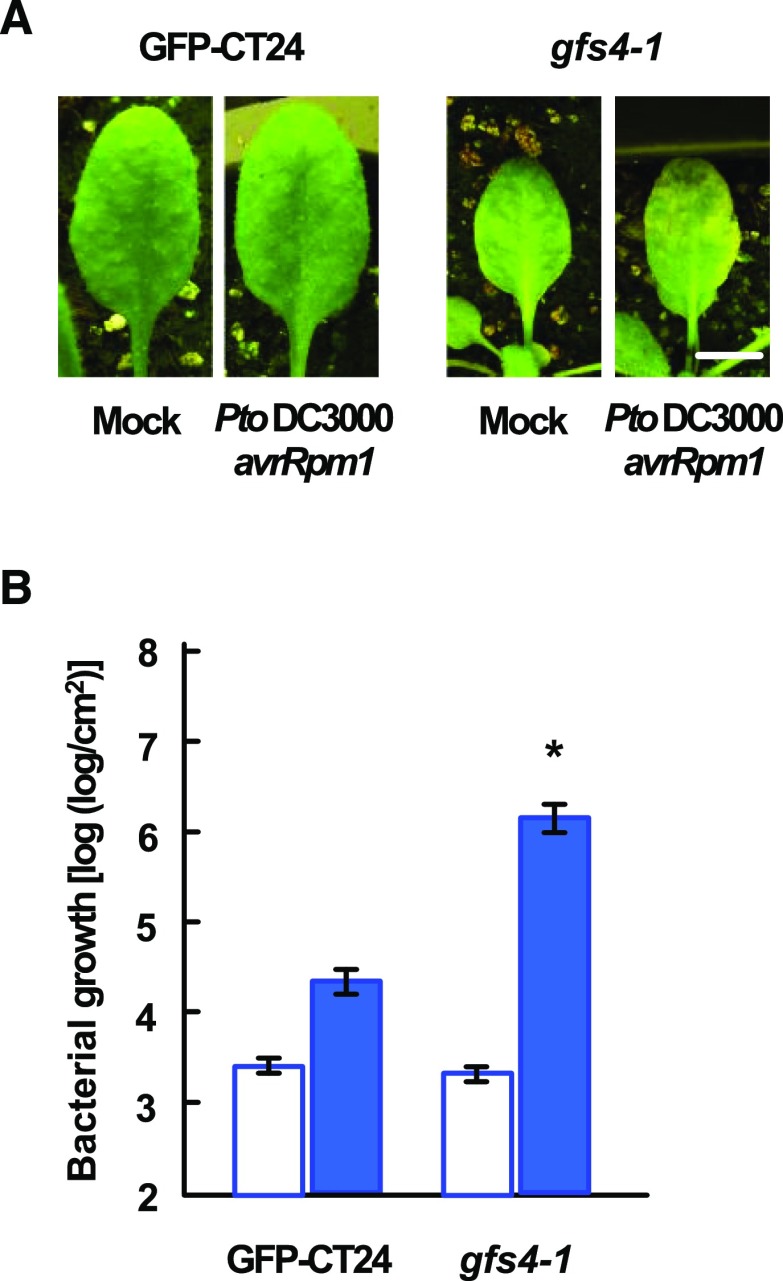
Sixty-four M2 lines of the Arabidopsis gfs mutant library (Col-0 background), previously identified as mutants that mis-sort the vacuolar protein GFP-CT24 out of cells (Fuji et al., 2007), were analyzed for their susceptibility to the avirulent bacterial strain, Pto DC3000, expressing AvrRpm1 effector protein (Pto DC3000 avrRpm1), which is recognized by the R protein RPM1 in Col-0 (Grant et al., 1995). We identified among gfs mutants a mutant that exhibited chlorotic symptoms within 5 d of bacterial inoculation (gfs4-1; Fig. 1A). To determine whether the susceptible phenotype of the mutant is associated with increased bacterial growth in the plant, we quantified the bacterial number in leaves 3 d after inoculation. The bacterial number in mutant leaves was approximately 100-fold higher than that in GFP-CT24 control leaves (Fig. 1B). These results suggest that the vacuolar protein sorting mutant has defects in ETI induced by infection with Pto DC3000 avrRpm1.
Figure 1.
gfs4-1 exhibits reduced resistance to Pto DC3000 avrRpm1. A, Leaves of GFP-CT24 and gfs4-1 plants 5 d after mock inoculation and inoculation with Pto DC3000 avrRpm1 (OD600 = 0.001). gfs4-1 leaves inoculated with Pto DC3000 avrRpm1 exhibit chlorotic symptoms. Bar = 0.5 cm. B, Bacterial growth immediately (white bars) and 3 d after (blue bars) inoculation with Pto DC3000 avrRpm1 (OD600 = 0.001) in the leaves of GFP-CT24 and gfs4-1. Each bar represents the mean and se of three independent experiments, each with six biological replicates. Asterisks indicate significant differences compared with GFP-CT24 plants (*P < 0.05, two-tailed t tests).
The AP-4 Subunit AP4B Is Important for ETI Mediated by RPM1 and RPS2 but Not by RPS4
DNA sequencing analysis previously identified the vacuolar sorting mutation in gfs4-1 (Supplemental Fig. S1) to be a single nucleotide substitution resulting in a premature stop codon in the At5g11490 gene, encoding the AP-4 subunit AP4B (Fuji et al., 2016). The AP-4 complex functions in receptor-mediated vacuolar protein sorting by recognizing VSR1, a Sorting receptor used in the targeting of seed storage proteins to protein storage vacuoles in Arabidopsis (Shimada et al., 2003; Robinson, 2004; Fuji et al., 2016).
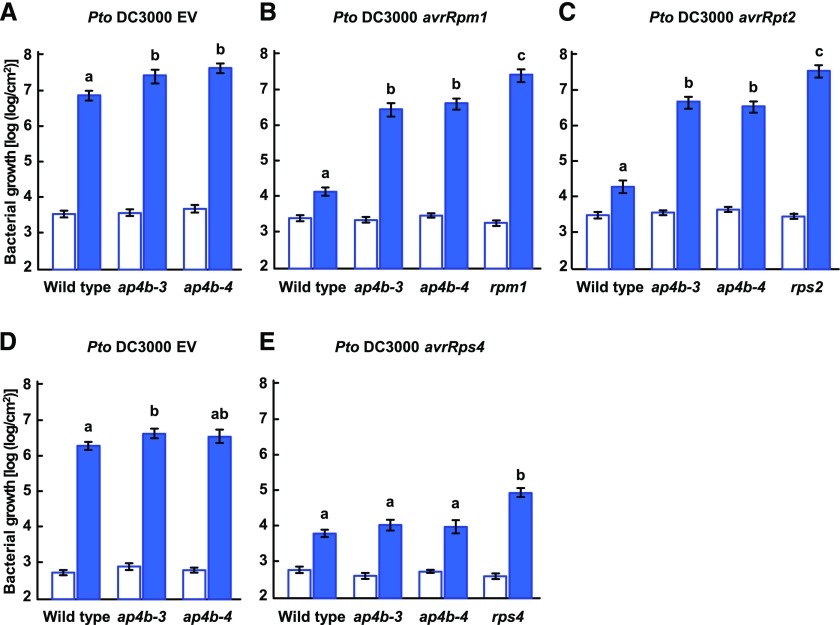
To investigate whether the defect in the AP4B gene is causal to the reduced ETI phenotype in gfs4-1, we examined whether resistance to Pto DC3000 avrRpm1 was reduced in two additional mutant alleles, ap4b-3/gfs4-3 and ap4b-4/gfs4-4, both of which are T-DNA insertion mutants (Supplemental Fig. S1; Fuji et al., 2016). We inoculated wild-type Col-0 and ap4b mutant plants with Pto DC3000 avrRpm1 and counted viable bacteria immediately and at 3 d after inoculation. The number of bacteria in ap4b-3 and ap4b-4 plants, like that in gfs4-1, was appromimately100-fold higher than that in the wild-type Col-0 plants at 3 d after inoculation (Fig. 2B). The growth of the bacteria in rpm1 plants was strongly enhanced, as expected for plants lacking the R protein (Fig. 2B). Thus, we conclude that the reduced ETI phenotype in gfs4-1 is caused by ap4b mutations. Bacterial growth of virulent strain Pto DC3000 carrying an empty vector (Pto DC3000 EV) after 3 d of inoculation was slightly higher in ap4b-3 and ap4b-4 plants than in wild-type plants (Fig. 2A). Since the effects of AP4B deficiency on the bacterial growth of Pto DC3000 EV were much smaller than that of Pto DC3000 avrRpm1, AP4B is important for RPM1-mediated ETI.
Figure 2.
Deficiency of AP4B compromised resistance to Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 but not to Pto DC3000 avrRps4. A to E, Bacterial growth immediately (white bars) and 3 d after (blue bars) inoculation with Pto DC3000 EV (OD600 = 0.001) (A), Pto DC3000 avrRpm1 (OD600 = 0.001) (B), Pto DC3000 avrRpt2 (OD600 = 0.001) (C), Pto DC3000 EV (OD600 = 0.0001) (D), and Pto DC3000 avrRps4 (OD600 = 0.0001) (E) in leaves of the indicated plant lines. A, B, and C were done at the same time, while D and E were done together at another time. Each bar represents the mean and se of three independent experiments, each with six biological replicates. Different letters indicate significant differences (P < 0.05, two-tailed t tests).
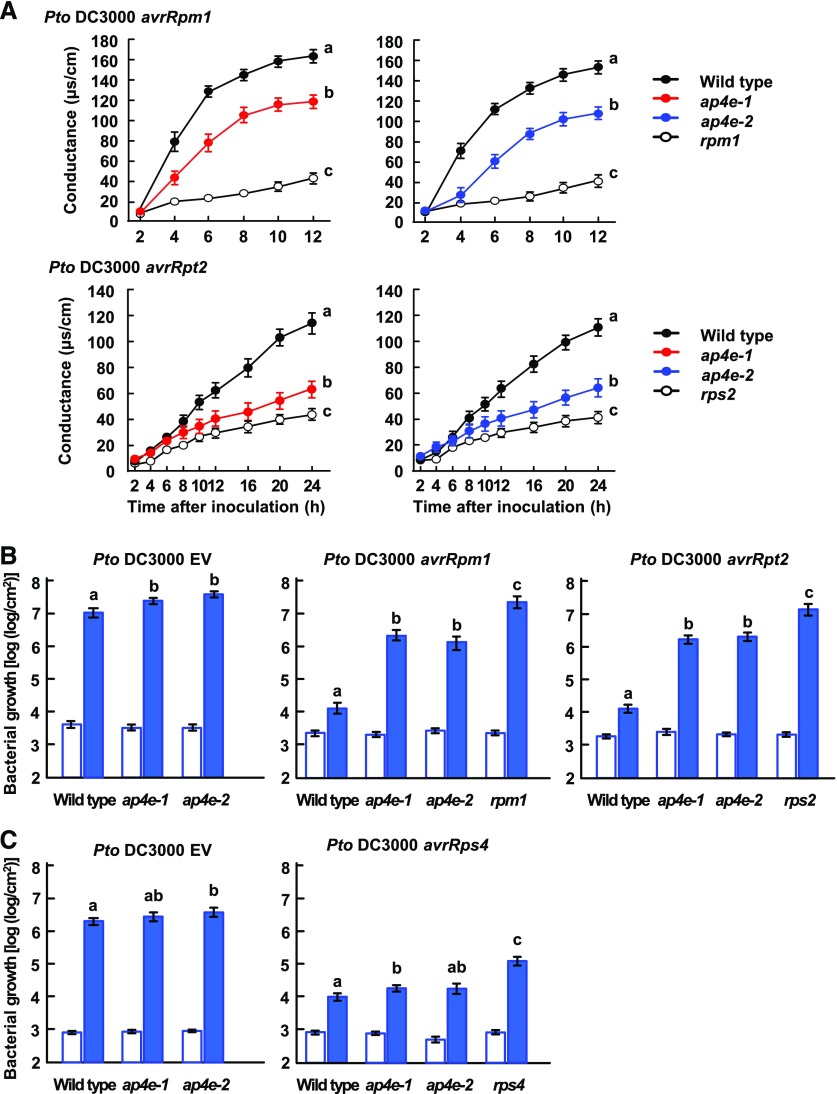
To determine whether the requirement of AP4B for ETI is specific to Pto DC3000 avrRpm1, we examined the bacterial growth of two additional bacterial strains, Pto DC3000 avrRpt2 expressing the AvrRpt2 effector protein and Pto DC3000 avrRps4 expressing the AvrRps4 effector protein. Pto DC3000 avrRpt2 and Pto DC3000 avrRps4 are recognized by the R proteins, RPS2 and RPS4, respectively, in Col-0 plants (Bent et al., 1994; Mindrinos et al., 1994). The number of Pto DC3000 avrRpt2 in ap4b-3 and ap4b-4 plants was approximately 100-fold higher than that in the wild-type Col-0 plants at 3 d after inoculation, but lower than the bacterial number in rps2 control plants (Fig. 2C). By contrast, no significant difference in the growth rates of Pto DC3000 avrRps4 was observed among ap4b-3, ap4b-4, and wild-type plants (Fig. 2E). The effects of AP4B deficiency on the bacterial growth of Pto DC3000 avrRps4 were similar to those of Pto DC3000 EV (Fig. 2, D and E). Taken together, these results indicate that AP4B is important for RPS2-mediated ETI, as well as RPM1-mediated ETI, but not for RPS4-mediated ETI.
AP4B Contributes to Hypersensitive Cell Death Induced by Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2
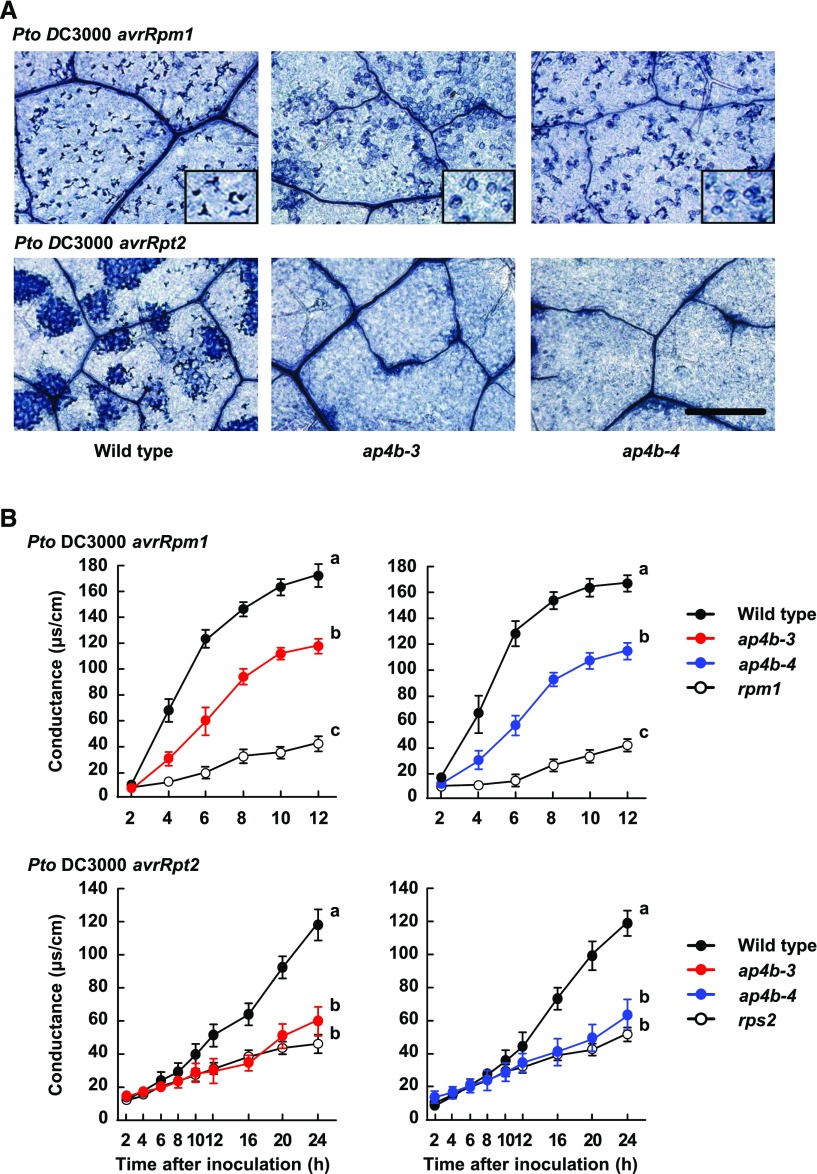
ETI is often associated with hypersensitive cell death (Dangl and Jones, 2001; Jones and Dangl, 2006). To determine whether AP4B deficiency affects hypersensitive cell death, we monitored cell death in wild-type Col-0 and ap4b plants using trypan blue staining at 12 h after inoculation with Pto DC3000 avrRpm1. The dead cells in wild-type plants exhibited a distinctive blue color under a light microscope (Fig. 3A, Pto DC3000 avrRpm1). The ap4b-3 and ap4b-4 mutant plants contained similar numbers of cells to wild-type plants in the field, but the blue color in the mutants was fainter, and the stained cells appeared to maintain their shape, in contrast to the stained cells in wild-type plants, which appeared to be shrunken (Fig. 3A, Pto DC3000 avrRpm1). These observations suggest that AP4B deficiency affects some aspects of the Pto DC3000 avrRpm1-induced hypersensitive cell death response. We also quantitatively monitored hypersensitive cell death based on the release of electrolytes from dead cells. Electrolyte leakage dramatically increased in wild-type plants after inoculation with Pto DC3000 avrRpm1, whereas the increase in electrolyte leakage was significantly lower in ap4b-3 and ap4b-4 plants than in the wild type but higher than in rpm1 plants (Fig. 3B, Pto DC3000 avrRpm1). These results indicate that AP4B deficiency leads to attenuated hypersensitive cell death associated with RPM1-mediated ETI.
Figure 3.
Hypersensitive cell death induced by Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 is reduced in ap4b-3 and ap4b-4 plants. A, Trypan blue staining of dead cells in the leaves of wild-type, ap4b-3, and ap4b-4 plants at 12 h after inoculation with Pto DC3000 avrRpm1 (OD600 = 0.001) and at 24 h after inoculation with Pto DC3000 avrRpt2 (OD600 = 0.002). Bar = 500 µm. B, Electrolyte leakage from dying and dead cells in the leaves of wild-type, ap4b-3, ap4b-4, rpm1, and rps2 plants inoculated with Pto DC3000 avrRpm1 (OD600 = 0.1) and Pto DC3000 avrRpt2 (OD600 = 0.1). Error bars indicate ses of three independent experiments, each with four biological replicates. Different letters indicate significant differences at 12 h (Pto DC3000 avrRpm1) and 24 h (Pto DC3000 avrRpt2) (P < 0.05, two-tailed t tests).
We further assessed hypersensitive cell death induced by infection with Pto DC3000 avrRpt2. Trypan blue staining revealed that Pto DC3000 avrRpt2-induced hypersensitive cell death was abolished in ap4b-3 and ap4b-4 mutant plants (Fig. 3A, Pto DC3000 avrRpt2). Similar results were obtained by quantitatively monitoring hypersensitive cell death based on electrolyte leakage. The rate of electrolyte leakage was much lower in Pto DC3000 avrRpt2-inoculated ap4b-3 and ap4b-4 plants than in wild-type plants but was the same as that in rps2 plants (Fig. 3B, Pto DC3000 avrRpt2). These results indicate that AP4B is required for hypersensitive cell death associated with RPS2-mediated ETI.
Pto DC3000 avrRps4-induced hypersensitive cell death is extremely weak in wild-type Col-0 plants (Gassmann et al., 1999; Tornero et al., 2002), which made it impossible to assess the effect of AP4B deficiency on hypersensitive cell death during RPS4-mediated ETI.
AP4B Is Required for Vacuolar Membrane Fusion to the Plasma Membrane
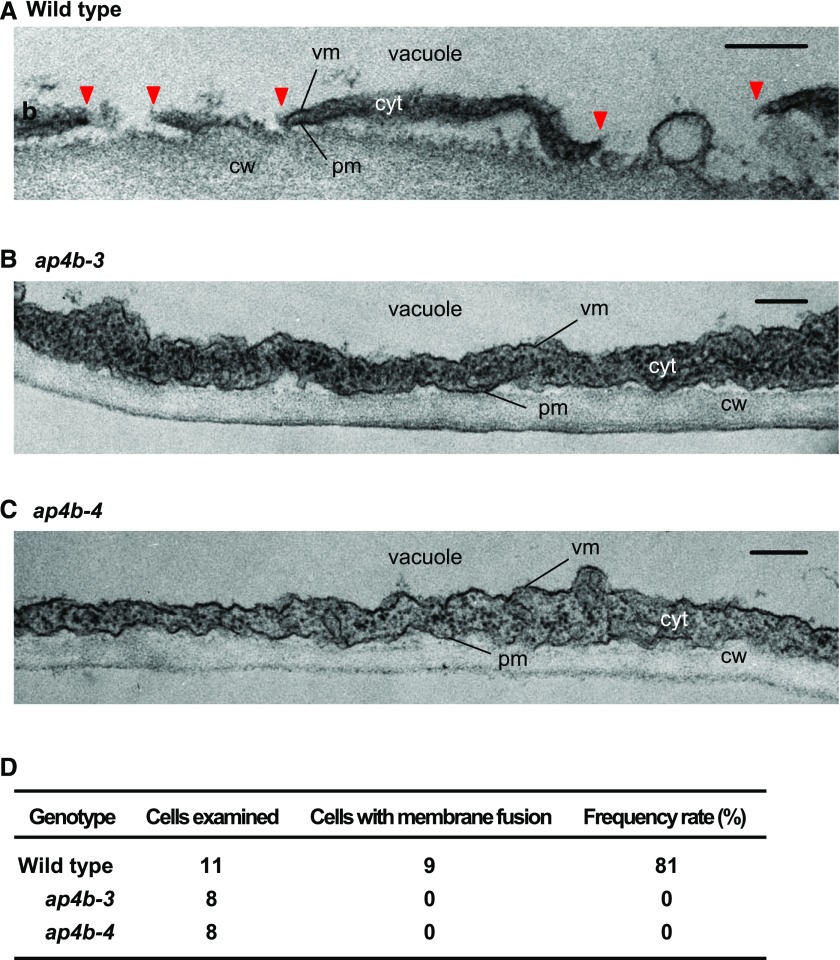
We previously showed that the large central vacuole fuses with the plasma membrane in response to avirulent bacterial infection, which is associated with hypersensitive cell death (Hatsugai et al., 2009). The strong inhibition of cell death in response to Pto DC3000 avrRpt2 infection in the ap4b mutants is reminiscent of the phenotype of RNAi ipba1 Arabidopsis lines in which the proteasome subunit gene PBA1 is silenced. The ipba1 lines fail to perform the membrane fusion in Pto DC3000 avrRpt2-inoculated leaves (Hatsugai et al., 2009). We investigated membrane fusion in the leaves of wild-type Col-0 and ap4b plants after inoculating them with Pto DC3000 avrRpt2. Vacuolar and plasma membrane fusion occurred in the leaves of wild-type Col-0 plants at 8 h after inoculation (Fig. 4A) and was detected in 81% of the examined cells (Fig. 4D), confirming a previous observation (Hatsugai et al., 2009). However, membrane fusion was not detected in the leaves of ap4b-3 and ap4b-4 plants (Fig. 4, B–D). These results indicate that AP4B is required for membrane fusion between the vacuolar membrane and the plasma membrane in response to Pto DC3000 avrRpt2 infection.
Figure 4.
Deficiency of AP4B suppresses fusion between the vacuolar membrane and plasma membrane in association with bacterial infection. A to C, Electron micrographs of the leaves of wild-type, ap4b-3, and ap4b-4 Arabidopsis plants at 8 h after inoculation with Pto DC3000 avrRpt2 (OD600 = 0.1). Membrane fusion between the plasma membrane and vacuolar membrane is indicated by red triangles. Bars = 200 nm. cw, cell wall; pm, plasma membrane; vm, vacuolar membrane; cyt, cytosol. D, Frequency rates of cells with fused membranes at 8 h after bacterial inoculation.
Another AP-4 Subunit AP4E Is Also Important for ETI
AP-4 is a heterotetrameric complex: its two large subunits are AP4B and AP4E. We therefore examined whether disrupting AP4E would lead to a compromised immune response, as was observed in the ap4b mutant. Arabidopsis T-DNA-insertion lines ap4e-1 and ap4e-2, with insertions in AP4E, also display abnormal vacuolar protein transport (Fuji et al., 2016). Electrolyte leakage assays following inoculation with Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 indicated that hypersensitive cell death was compromised in ap4e-1 and ap4e-2 (Fig. 5A). Consistent with this observation, the ap4e-1 and ap4e-2 plants exhibited substantially increased bacterial growth of Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 compared with wild-type plants (Fig. 5B, Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2). The growth rate of Pto DC3000 EV was slightly higher in ap4e-1 and ap4e-2 plants than in wild-type plants (Fig. 5B, Pto DC3000 EV). Since the effects of AP4E deficiency on the bacterial growth of Pto DC3000 EV were much smaller than that of Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2, AP4E is important for resistance to Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 infections. By contrast, 3 d after inoculation, the growth rates of Pto DC3000 avrRps4 in ap4e-1 and ap4e-2 plants were not different from that in the wild type plants (Fig. 5C, Pto DC3000 avrRps4). AP4E deficiency on the bacterial growth of Pto DC3000 avrRps4 were comparable to that of Pto DC3000 EV (Fig. 5C). Taken together, these results indicate that AP4E is involved in both RPM1-mediated and RPS2-mediated ETI but not involved in RPS4-mediated ETI.
Figure 5.
Both resistance and hypersensitive cell death in response to Pto DC3000 avrRpm1 and Pto DC3000 avrRpt2 are compromised in ap4e-1 and ap4e-2 plants. A, Electrolyte leakage from dying and dead cells in the leaves of wild-type, ap4e-1, and ap4e-2 plants inoculated with Pto DC3000 avrRpm1 (OD600 = 0.1) and Pto DC3000 avrRpt2 (OD600 = 0.1). Error bars indicate ses of three independent experiments, each with four biological replicates. Different letters indicate significant differences at 12 h (Pto DC3000 avrRpm1) and 24 h (Pto DC3000 avrRpt2) (P < 0.05, two-tailed t tests). B, Bacterial growth immediately (white bars) and 3 d after (blue bars) inoculation with Pto DC3000 avrRpm1 (OD600 = 0.001), Pto DC3000 avrRpt2 (OD600 = 0.001), and Pto DC3000 EV (OD600 = 0.001) in leaves of wild type, ap4e-1, and ap4e-2. C, Bacterial growth immediately (white bars) and 3 days after (blue bars) inoculation with Pto DC3000 EV (OD600 = 0.0001) and Pto DC3000 avrRps4 (OD600 = 0.0001) in leaves of the indicated plant lines. Each bar represents the mean and se of three independent experiments, each with six biological replicates. Different letters indicate significant differences (P < 0.05, two-tailed t tests).
AP4B and AP4E Are Weakly Involved in PTI
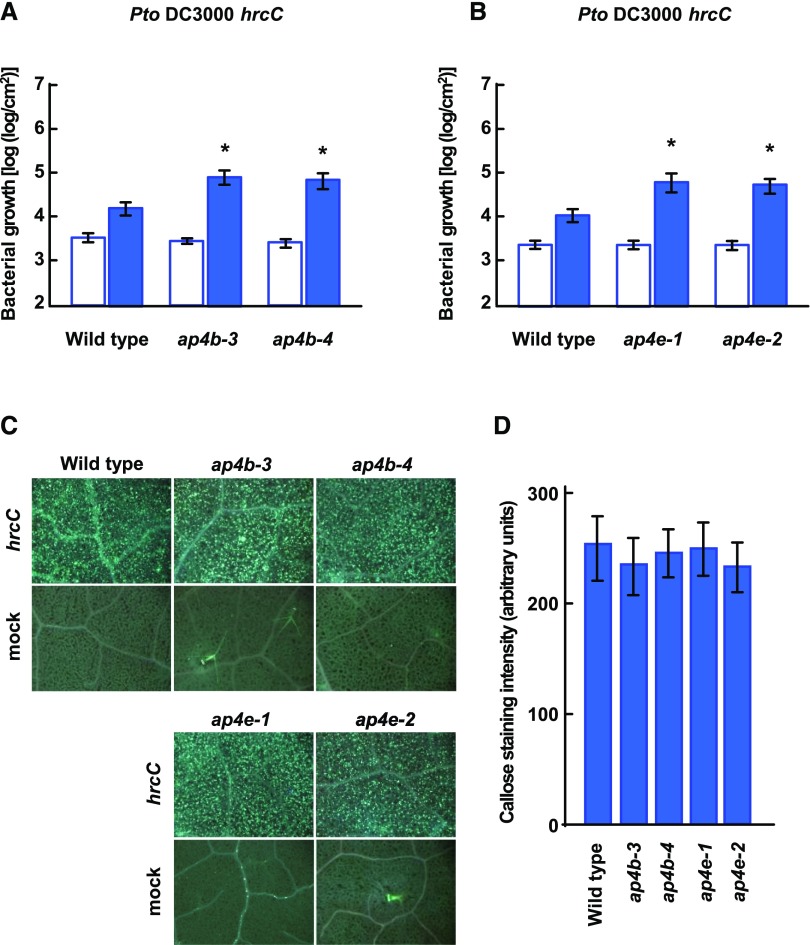
The enhanced bacterial growth of Pto DC3000 EV in the ap4b and ap4e mutants suggested that AP4B and AP4E are involved in PTI as well as ETI. To test this hypothesis, we inoculated wild-type Col-0, ap4b, and ap4e plants with Pto DC3000 hrcC and counted viable bacteria immediately and 3 d after inoculation. The hrcC bacterial strain cannot secrete type III effectors due to a lack of a type III secretion system component, resulting in the activation of PTI in Arabidopsis (Hauck et al., 2003). The ap4b and ap4e plants exhibited slightly but significantly higher growth of Pto DC3000 hrcC compared to wild-type plants (Fig. 6, A and B). These results indicate that AP4B and AP4E are weakly involved in PTI.
Figure 6.
Deficiency of AP4B or AP4E increases bacterial growth of Pto DC3000 hrcC, whereas it does not affect callose deposition. A and B, Bacterial growth immediately (white bars) and 3 d after (blue bars) inoculation with Pto DC3000 hrcC (OD600 = 0.001) in leaves of the indicated plant lines. Each bar represents the mean and se of three independent experiments, each with six biological replicates. Asterisks indicate significant differences compared with wild-type plants (*P < 0.05, two-tailed t tests). C, Callose deposition in leaves of the indicated plant lines inoculated with Pto DC3000 hrcC (OD600 = 0.1) (hrcC) and water (mock) detected by aniline blue staining at 12 h after inoculation. Bar = 500 µm. D, Quantification of callose staining intensity. Each bar represents the mean and se from five stained regions in three different leaves.
Pto DC3000 hrcC-activated callose deposition is a response associated with PTI. To examine the involvement of AP4B and AP4E in callose deposition, we inoculated wild-type Col-0, ap4b, and ap4e plants with Pto DC3000 hrcC and stained them for callose. Pto DC3000 hrcC inoculation induced a large number of highly localized callose deposits in the leaves of ap4b and ap4e plants as well as wild-type plants (Fig. 6, C and D). No callose deposition was observed in mock-treated plants (Fig. 6C). These results indicate that the beta and ε subunits of the AP-4 complex are not required for the callose deposition response.
DISCUSSION
AP-4 Complex Is Required for Vacuolar Membrane Fusion to the Plasma Membrane during ETI
The ap4b and ap4e mutations substantially compromised ETI mediated by RPM1 and RPS2, which is involved HR cell death. By contrast, RPS4-mediated ETI, which is independent of HR cell death, was not affected by ap4b and ap4e mutations. These results suggest that the AP-4 complex is involved in cell death-associated immunity. The cell death-associated immune response is in some cases accompanied by vacuolar and plasma membrane fusion. These observations are consistent with the notion that AP-4 is required for membrane fusion. However, the molecular role of the AP-4 complex in membrane fusion remains unclear. The AP-4 complex may be involved in vacuolar protein sorting of immune-associated cargo proteins responsible for membrane fusion. The cargo proteins are likely vacuolar membrane proteins and soluble proteins recognized by VSR homologs, because we previously found that the AP-4 complex functions in the transport of VSR1 to the target membrane and is required for VSR1-mediated vacuolar protein transport in seed cells (Fuji et al., 2016).
Fusion of the cellular membrane with the vacuolar membrane often requires soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (Zhang et al., 2014; Uemura and Ueda, 2014). In the animal immune response, fusion between the lysosome and plasma membrane occurs in activated cytotoxic T lymphocyte and natural killer cells to induce apoptosis/PCD (Blott and Griffiths, 2002; Luzio et al., 2007). This membrane fusion is mediated by a lysosomal SNARE protein complex containing vesicle-associated membrane protein 7 (VAMP7), a member of the R-SNARE family (Blott and Griffiths, 2002; Luzio et al., 2007; Marcet-Palacios et al., 2008; Chaineau et al., 2009). Given the evidence suggesting that SNARE proteins are targeted to the lysosome membrane through interactions with APs, including AP-4 (Martinez-Arca et al., 2003; Bennett et al., 2008; Kent et al., 2012), the AP-4 complex in plants may guide SNARE proteins to the vacuole, where they probably participate in the fusion of vacuolar and plasma membranes in response to pathogen infection. Interestingly, OsVAMP7, a rice (Oryza sativa) SNARE localized to the vacuolar membrane, is suggested to play a role in resistance to blast disease (Sugano et al., 2016). In addition, SNARE proteins involved in plant immune responses have also been reported, although they are mostly associated with the plasma membrane (Collins et al., 2003; Kalde et al., 2007; Uemura et al., 2012). Future identification of the cargo proteins of the AP-4 complex may provide clues about the molecular mechanism underlying membrane fusion and its regulation of HR cell death during ETI.
AP-4 Complex-Dependent Transport of Vacuolar Proteins in Plant Immunity
Plant vacuoles contain immune-related proteins as well as hydrolases and lipases (De, 2000; Carter et al., 2004). Some of these proteins are transported into vacuoles as proprotein precursors, where they are proteolytically processed to produce the respective mature/active forms (Hara-Nishimura et al., 1991; Kunze et al., 1998; Rojo et al., 2003; Shimada et al., 2003; Yamada et al., 2005). Vacuolar and plasma membrane fusion causes the discharge of accumulated mature/active vacuolar proteins into the extracellular space, leading to the suppression of extracellular bacterial growth and hypersensitive cell death (Hatsugai et al., 2009). In ap4 mutants, vacuolar proteins may not accumulate sufficiently in the vacuole, because their proprotein precursors are not efficiently transported into vacuoles (Fuji et al., 2016). The resulting low amounts of such vacuolar proteins could result in loss of cell death and immunity to pathogen infection.
AP-4 complex-regulated vacuolar protein sorting is initiated after recognition of the VSR1 sorting receptor (Fuji et al., 2016). A homolog of VSR1 is transcriptionally induced during plant immune responses in Arabidopsis (Wang et al., 2005), although its functional relevance to plant immunity is unknown. VSR homolog(s), which could be recognized by AP-4, might be necessary to transport immune-related proteins to vacuoles, where they undergo maturation or activation.
A Possible Function of AP-4 Complex in ETI Signaling
RPM1 and RPS2 are localized to plasma membrane, while RPS4 is localized to the cytoplasm and nucleus (Axtell and Staskawicz, 2003; Mackey et al., 2003; Wirthmueller et al., 2007). This study shows that AP-4 complex influences the ETI mediated by plasma membrane-localized R proteins. This is consistent with our previous report that the AP-2 subunit AP2M is required for both RPM1-mediated ETI and RPS2-mediated ETI, but not for RPS4-mediated ETI (Hatsugai et al., 2016). AP2M plays a central role in clathrin-mediated endocytotic pathway from the plasma membrane to the vacuole (Di Rubbo et al., 2013; Fan et al., 2013; Kim et al., 2013; Yamaoka et al., 2013). These results suggest that the ETI-related immune-signaling components undergo endocytic internalization. Thus, AP-4 complex might be involved in vacuolar trafficking of immune-signaling components through the TGN/early endosome for degradation or turnover, resulting in efficient ETI signaling.
AP-4 Complex Is Likely a Minor Component of PTI
Fusion between the vacuolar membrane and the plasma membrane is not observed during the basal defense response (Hatsugai et al., 2009), which is consistent with the finding that PTI is generally not associated with cell death. The ap4b and ap4e mutants exhibited enhanced bacterial growth of Pto DC3000 hrcC. By contrast, callose deposition induced by this strain was intact in ap4b and ap4e mutant plants. These results indicate that although AP-4 is involved in PTI, the extent of its involvement is limited. The transport of callose to the extracellular space is mediated by the ADP ribosylation factor guanine nucleotide exchange factor (Nomura et al., 2006; Nielsen et al., 2012). A major aspect of PTI, including callose deposition, is mediated by extracellular transport, which is not affected by deficiencies in AP-4 (Hückelhoven, 2007; Malinovsky et al., 2014; Fuji et al., 2016). These findings are consistent with the observation that AP-4 deficient mutants did not exhibit a defect in extracellular transport (Fuji et al., 2016).
Taken together, our results provide substantive evidence to show that the AP-4 complex is required for ETI-associated HR cell death but is likely a minor component of PTI.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was the background line for all plants used in this study. Arabidopsis gfs lines (Fuji et al., 2007) ap4b-3/gfs4-3 (SAIL_796A10; Fuji et al., 2016), ap4b-4/gfs4-4 (SAIL_781H01; Fuji et al., 2016), ap4e-1 (SAIL_866C01; Fuji et al., 2016), ap4e-2 (SAIL_60E03; Fuji et al., 2016), GFP-CT24 (Nishizawa et al., 2003), rpm1-3 (Grant et al., 1995), rps2-101C (Mindrinos et al., 1994), and rps4 (SALK_057697; Wirthmueller et al., 2007) were described previously. The Arabidopsis plants were grown in a controlled environment at 22°C with a 12-h photoperiod.
DNA Sequencing
DNA was isolated from F2 progeny. The nucleotide sequences of both strands were determined using an ABI Prism Big Dye Terminator Cycle Sequence Reaction Kit (Applied Biosystems) and a DNA sequencer (model 3100-Avant Genetic Analyzer; Applied Biosystems).
Bacterial Strains and Pathology Tests
Pseudomonas syringae pv. tomato virulent strain DC3000 carrying the empty vector pVSP61 (Pto DC3000 EV), congenic avirulent strains (Pto DC3000 expressing avrRpm1, avrRpt2 or avrRps4), and Pto DC3000 hrcC were used in this study. Bacteria were cultured in King’s B medium, washed twice in 10 mm MgCl2, and resuspended at OD600 = 0.001 or 0.0001 for plant inoculation for the growth assays and trypan blue staining and at OD600 = 0.1 for the electrolyte leakage assay and electron microscopic analysis. Bacterial suspensions were inoculated into the abaxial sides of 4- to 5-week-old Arabidopsis leaves using needleless syringes. Bacterial growth was monitored as described previously (Hatsugai et al., 2009).
Trypan Blue Staining
Inoculated leaves were boiled for approximately 1 min in staining solution (1 mL lactic acid, 1 mL glycerol, 1 mL water-saturated phenol, 1 mL water, and 1 mg trypan blue) and incubated overnight at room temperature. The samples were then decolorized in 2.5 g/mL chloral hydrate for at least 1 d.
Electrolyte Leakage Assay
Electrolyte leakage from dying and dead cells was measured essentially as described (Hatsugai et al., 2009). Four disks with a 7.5-mm diameter were dissected from leaves immediately after bacterial inoculation, floated in 2 mL distilled water for 30 min, transferred to 2 mL fresh distilled water, and incubated at 22°C. Water conductance was measured with an electrical conductivity meter (B-173, Horiba).
Callose Deposition
Callose staining was performed essentially as described (Adam and Somerville, 1996). Leaves were vacuum-infiltrated with alcoholic lactophenol (one volume of phenol/glycerol/lactic acid/water [1:1:1:1] and two volumes of ethanol) and incubated at 65°C for 30 to 60 min. The leaves were transferred to fresh alcoholic lactophenol and incubated for an additional 24 h until they were completely cleared of chlorophyll. The leaf samples were rinsed in 50% ethanol, 20% ethanol, 10% ethanol, and water, stained for 30 min in 150 mm K2HPO4 (pH 9.5) containing 0.01% aniline blue, and observed using ultraviolet illumination at 340- to 360-nm excitation and 450- to 470-nm emission wavelengths under an Eclipse E600 microscope (Nikon). Grayscale images of callose-stained leaves were used to quantify the signal intensity using Fiji/ImageJ software ver. 1.47q (Schindelin et al., 2012).
Electron Microscopic Analysis
Arabidopsis leaves that were infected with Pto DC3000 avrRpt2 were vacuum-infiltrated for 1 h in fixative, dehydrated, and embedded in Epon as described previously (Hatsugai et al., 2009). Ultra-thin sections were examined under a transmission electron microscope (model 1200EX; JEOL) at 80 kV.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under accession numbers AP4B (At5g11490) and AP4E (At1g31730).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Arabidopsis plants used in this study.
Acknowledgments
We are grateful to the ABRC at Ohio State University for providing Arabidopsis T-DNA insertion mutant seeds.
Footnotes
Articles can be viewed without a subscription.
References
- Adam L, Somerville SC (1996) Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J 9: 341–356 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Staskawicz BJ (2003) Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112: 369–377 [DOI] [PubMed] [Google Scholar]
- Bennett N, Letourneur F, Ragno M, Louwagie M (2008) Sorting of the v-SNARE VAMP7 in Dictyostelium discoideum: a role for more than one Adaptor Protein (AP) complex. Exp Cell Res 314: 2822–2833 [DOI] [PubMed] [Google Scholar]
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994) RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265: 1856–1860 [DOI] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3: 122–131 [DOI] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaineau M, Danglot L, Galli T (2009) Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett 583: 3817–3826 [DOI] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL (2011) Programmed cell death in the plant immune system. Cell Death Differ 18: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu J-L, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al. (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- De D. (2000) Plant cell vacuoles. Collingwood, Australia, Csiro Publishing [Google Scholar]
- Di Rubbo S, Irani NG, Kim SY, Xu ZY, Gadeyne A, Dejonghe W, Vanhoutte I, Persiau G, Eeckhout D, Simon S, et al. (2013) The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 25: 2986–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Hao H, Xue Y, Zhang L, Song K, Ding Z, Botella MA, Wang H, Lin J (2013) Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development 140: 3826–3837 [DOI] [PubMed] [Google Scholar]
- Fuji K, Shimada T, Takahashi H, Tamura K, Koumoto Y, Utsumi S, Nishizawa K, Maruyama N, Hara-Nishimura I (2007) Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 19: 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji K, Shirakawa M, Shimono Y, Kunieda T, Fukao Y, Koumoto Y, Takahashi H, Hara-Nishimura I, Shimada T (2016) The adaptor complex AP-4 regulates vacuolar protein sorting at trans-Golgi network by interacting with VACUOLAR SORTING RECEPTOR 1. Plant Physiol 170: 211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Hinsch ME, Staskawicz BJ (1999) The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant J 20: 265–277 [DOI] [PubMed] [Google Scholar]
- Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL (1995) Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269: 843–846 [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Hatsugai N (2011) The role of vacuole in plant cell death. Cell Death Differ 18: 1298–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I, Inoue K, Nishimura M (1991) A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett 294: 89–93 [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Hara-Nishimura I (2010) Two vacuole-mediated defense strategies in plants. Plant Signal Behav 5: 1568–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Hillmer R, Yamaoka S, Hara-Nishimura I, Katagiri F (2016) The μ Subunit of Arabidopsis Adaptor Protein-2 Is Involved in Effector-Triggered Immunity Mediated by Membrane-Localized Resistance Proteins. Mol Plant Microbe Interact 29: 345–351 [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, Hara-Nishimura I (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev 23: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305: 855–858 [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Yamada K, Goto-Yamada S, Hara-Nishimura I (2015) Vacuolar processing enzyme in plant programmed cell death. Front Plant Sci 6: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck P, Thilmony R, He SY (2003) A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA 100: 8577–8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Goh T, Hayashi T, Kutsuna N, Kadota Y, Hasezawa S, Sano T, Kuchitsu K (2007) Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol 48: 1414–1425 [DOI] [PubMed] [Google Scholar]
- Hückelhoven R. (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45: 101–127 [DOI] [PubMed] [Google Scholar]
- Iglesias A, Meins F (2000) Vacuoles and plant defense. Annual Plant Reviews 5: 112–132 [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kalde M, Nühse TS, Findlay K, Peck SC (2007) The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci USA 104: 11850–11855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent HM, Evans PR, Schäfer IB, Gray SR, Sanderson CM, Luzio JP, Peden AA, Owen DJ (2012) Structural basis of the intracellular sorting of the SNARE VAMP7 by the AP3 adaptor complex. Dev Cell 22: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Xu ZY, Song K, Kim DH, Kang H, Reichardt I, Sohn EJ, Friml J, Juergens G, Hwang I (2013) Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell 25: 2970–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze I, Kunze G, Bröker M, Manteuffel R, Meins F Jr, Müntz K (1998) Evidence for secretion of vacuolar α-mannosidase, class I chitinase, and class I β-1,3-glucanase in suspension cultures of tobacco cells. Planta 205: 92–99 [DOI] [PubMed] [Google Scholar]
- Kuroyanagi M, Yamada K, Hatsugai N, Kondo M, Nishimura M, Hara-Nishimura I (2005) Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem 280: 32914–32920 [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8: 622–632 [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112: 379–389 [DOI] [PubMed] [Google Scholar]
- Malinovsky FG, Fangel JU, Willats WG (2014) The role of the cell wall in plant immunity. Front Plant Sci 5: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Palacios M, Odemuyiwa SO, Coughlin JJ, Garofoli D, Ewen C, Davidson CE, Ghaffari M, Kane KP, Lacy P, Logan MR, et al. (2008) Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem Biophys Res Commun 366: 617–623 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, et al. (2003) A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc Natl Acad Sci USA 100: 9011–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindrinos M, Katagiri F, Yu GL, Ausubel FM (1994) The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78: 1089–1099 [DOI] [PubMed] [Google Scholar]
- Munch D, Teh O-K, Malinovsky FG, Liu Q, Vetukuri RR, El Kasmi F, Brodersen P, Hara-Nishimura I, Dangl JL, Petersen M, et al. (2015) Retromer contributes to immunity-associated cell death in Arabidopsis. Plant Cell 27: 463–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen ME, Feechan A, Böhlenius H, Ueda T, Thordal-Christensen H (2012) Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci USA 109: 11443–11448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa K, Maruyama N, Satoh R, Fuchikami Y, Higasa T, Utsumi S (2003) A C-terminal sequence of soybean beta-conglycinin alpha’ subunit acts as a vacuolar sorting determinant in seed cells. Plant J 34: 647–659 [DOI] [PubMed] [Google Scholar]
- Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY (2006) A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313: 220–223 [DOI] [PubMed] [Google Scholar]
- Robinson MS. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol 14: 167–174 [DOI] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 7389–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Koumoto Y, Li L, Yamazaki M, Kondo M, Nishimura M, Hara-Nishimura I (2006) AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol 47: 1187–1194 [DOI] [PubMed] [Google Scholar]
- Sugano S, Hayashi N, Kawagoe Y, Mochizuki S, Inoue H, Mori M, Nishizawa Y, Jiang C-J, Matsui M, Takatsuji H (2016) Rice OsVAMP714, a membrane-trafficking protein localized to the chloroplast and vacuolar membrane, is involved in resistance to rice blast disease. Plant Mol Biol 91: 81–95 [DOI] [PubMed] [Google Scholar]
- Teh OK, Hatsugai N, Tamura K, Fuji K, Tabata R, Yamaguchi K, Shingenobu S, Yamada M, Hasebe M, Sawa S, et al. (2015) BEACH-domain proteins act together in a cascade to mediate vacuolar protein trafficking and disease resistance in Arabidopsis. Mol Plant 8: 389–398 [DOI] [PubMed] [Google Scholar]
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F (2010) Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Uemura T, Kim H, Saito C, Ebine K, Ueda T, Schulze-Lefert P, Nakano A (2012) Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc Natl Acad Sci USA 109: 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Ueda T (2014) Plant vacuolar trafficking driven by RAB and SNARE proteins. Curr Opin Plant Biol 22: 116–121 [DOI] [PubMed] [Google Scholar]
- Wang D, Weaver ND, Kesarwani M, Dong X (2005) Induction of protein secretory pathway is required for systemic acquired resistance. Science 308: 1036–1040 [DOI] [PubMed] [Google Scholar]
- Wirthmueller L, Zhang Y, Jones JD, Parker JE (2007) Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol 17: 2023–2029 [DOI] [PubMed] [Google Scholar]
- Yamada K, Shimada T, Nishimura M, Hara-Nishimura I (2005) A VPE family supporting various vacuolar functions in plants. Physiol Plant 123: 369–375 [Google Scholar]
- Yamaoka S, Shimono Y, Shirakawa M, Fukao Y, Kawase T, Hatsugai N, Tamura K, Shimada T, Hara-Nishimura I (2013) Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 25: 2958–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2008) Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol 49: 142–156 [DOI] [PubMed] [Google Scholar]
- Yang Y, Shah J, Klessig DF (1997) Signal perception and transduction in plant defense responses. Genes Dev 11: 1621–1639 [DOI] [PubMed] [Google Scholar]
- Zhang C, Hicks GR, Raikhel NV (2014) Plant vacuole morphology and vacuolar trafficking. Front Plant Sci 5: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhar J, Muñoz A, Rojo E (2010) Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. Plant J 64: 577–588 [DOI] [PubMed] [Google Scholar]