The role of 18F‐FDG PET in the diagnostic algorithm of patients with advanced entero‐pancreatic neuroendocrine neoplasms is still to be established. This article reports on the ability of 18F‐FDG PET to identify cases of advanced entero‐pancreatic neuroendocrine neoplasms with more aggressive and unfavorable clinical outcomes.
Keywords: 18F‐fluorodeoxyglucose positron emission tomography, Neuroendocrine tumors, Prognosis, Clinical usefulness, Diagnosis
Abstract
Background.
The role of 18F‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET) in the diagnostic algorithm of entero‐pancreatic neuroendocrine neoplasms (EP NENs) is unclear because most available data derive from heterogeneous populations in terms of tumor biology and disease status at time of examination. The aim of this study was to determine the ability of 18F‐FDG PET to identify patients with more aggressive disease among those with advanced EP NENs.
Subjects, Materials, and Methods. Patients with advanced EP NENs and known disease status (progressive disease [PD] or stable disease [SD]) according to imaging procedures, who received 18F‐FDG PET and computed tomography scans during a time frame of 1 month, were included.
Results.
A total of 93 patients, including 69 patients with pancreatic NENs and 24 patients with small‐intestine NENs, were included. At the time of study entry, 64 patients (68.8%) had PD, and the remaining 29 patients (31.2%) had SD. A total of 62 patients (66.7%) had positive 18F‐FDG PET, whereas 18F‐FDG PET was negative in the remaining 31 patients (33.3%). Overall, 18F‐FDG PET sensitivity and specificity to detect PD were 90.6% and 86.2%, respectively, resulting in a diagnostic accuracy of 89.2%. A positive 18F‐FDG PET was significantly associated with PD at the time of study entry (p < .0001 at multivariate analysis). Although a higher proportion of 18F‐FDG PET‐positive examinations were observed in patients with higher tumor grade (p = .01), 53.8% of patients with grade 1 neuroendocrine tumors (NETs) had positive 18F‐FDG PET, and 37.5% of patients with grade 2 NETs had negative 18F‐FDG PET. Overall survival was significantly shorter in 18F‐FDG PET‐positive patients (median: 60 months) in comparison with 18F‐FDG PET‐negative patients (median not reached; p = .008).
Conclusion.
18F‐FDG PET has a high diagnostic accuracy to identify progression of disease with unfavorable clinical outcome in patients with advanced EP NENs. Knowledge of disease status and G grading are key factors for physicians to better select patients for whom 18F‐FDG PET is clinically useful.
Implications for Practice.
The findings of the present study may help physicians dealing with advanced neuroendocrine neoplasms to select patients for whom 18F‐fluorodeoxyglucose positron emission tomography is useful to predict poor clinical outcome.
摘要
背景.18F‐氟脱氧葡萄糖正电子发射断层扫描(18F‐FDG PET)在肠胰神经内分泌肿瘤(EP NEN)诊断算法中的作用尚不清楚, 因为就检查时的肿瘤生物学和疾病状态而言, 大多数可获得的数据来源于异质性群体。本研究的目的是确定18F‐FDG PET能够在晚期EP NEN患者中鉴别出更具侵袭性疾病的能力。
受试者、材料和方法.纳入利用影像学方法确定具有晚期EP NEN和已知疾病状态[疾病进展(PD)或疾病稳定(SD)]的患者, 这些患者在1个月的时间范围内接受18F‐FDG PET和计算机断层扫描。
结果.共纳入93例患者, 其中包括69例胰腺NEN患者和24例小肠NEN患者。研究入组时, 64例患者(68.8%)PD, 其余29例患者(31.2%)SD。总共62例患者(66.7%)18F‐FDG PET为阳性, 其余31例患者(33.3%)18F‐FDG PET为阴性。总体而言, 18F‐FDG PET诊断PD的灵敏度和特异性分别为90.6%和86.2%, 诊断准确率为89.2%。研究入组时, 18F‐FDG PET阳性与PD显著相关(多因素分析p<0.000 1)。尽管在肿瘤分级较高的患者中, 18F‐FDG PET阳性检查的比例较高(p=0.01), 但1级神经内分泌肿瘤(NET)患者中有53.8%为18F‐FDG PET阳性, 2级NET患者中有37.5%为18F‐FDG PET阴性。与18F‐FDG PET阴性患者(未达到中位数;p=0.008)相比, 18F‐FDG PET阳性患者的总生存期明显更短(中位数:60个月)。
结论.18F‐FDG PET用于鉴别晚期EP NEN患者不良临床预后的疾病进展时具有较高的诊断准确率。了解疾病状态和G分级是医生在临床上更好地选择可获益于18F‐FDG PET的患者的关键因素。
对临床实践的启示:本研究的结果可能有助于医生处理晚期神经内分泌肿瘤, 利用18F‐氟脱氧葡萄糖正电子发射断层扫描选择可能预测不良临床结局的患者。
Introduction
Entero‐pancreatic neuroendocrine neoplasms (EP NENs) are rare tumors arising from neuroendocrine cells in the pancreas and in the intestinal tract. Although their incidence is low (6.9 cases per 100,000), their prevalence is relatively high (48 cases per 100,000) due to patients’ long survival [1].
Several factors affect their prognosis, including the specific primary tumor site, histological features, tumor grading according to the World Health Organization (WHO) classification, degree of differentiation, and tumor burden [2], [3]. The major prognostic factor is Ki67, which is the basis of the WHO classification and the European Neuroendocrine Tumor Society (ENETS) G‐grading system [4], [5], [6], [7], [8].
Noninvasive functional imaging with 18F‐fluorodeoxyglucose (18F‐FDG) positron emission tomography (PET) has been proposed as an alternative to tissue sampling for the determination of the aggressiveness of tumors [9] and has shown prognostic value in several kinds of cancer other than NENs. In fact, it is commonly used for initial diagnosis, as well as to assess treatment efficacy in patients with pulmonary cancer [10], Hodgkin's lymphoma [11], [12], gastrointestinal stromal tumors [13], and colorectal cancer [14].
18F‐FDG PET is not routinely used in NENs, because they are generally slow growing and, accordingly, have a low glycolytic activity, which may result in low diagnostic accuracy. However, previous studies on small groups of patients with EP NENs suggest that 18F‐FDG PET might be of value for detecting more aggressive tumors with high proliferative Ki67 index [15], [16]. A role for 18F‐FDG PET, alone or in combination with 68Ga PET, has also been proposed, with conflicting results, in some series of NENs with a more aggressive clinical course [17], [18], [19], [20], [21].
However, due to the heterogeneity of the findings reported in the above studies and the lack of data focused on its diagnostic accuracy according to disease behavior (progressive disease [PD] or stable disease [SD]), the role of 18F‐FDG PET in the diagnostic algorithm of patients with advanced EP NENs is still to be established.
Therefore, the present study aimed at investigating the ability of 18F‐FDG PET to identify, among patients with advanced EP NENs, disease progression and cases with more aggressive and unfavorable clinical outcomes.
Subjects, Materials, and Methods
Study Design
All consecutive patients with advanced, unresectable, histologically proven diagnosis of EP NENs, seen at the two participating centers (Sant'Andrea Hospital Center, Rome, and San Raffaele Hospital‐Negrar Hospital, Milan and Negrar) from January 2011 to January 2016, were evaluated as candidates for this study. Inclusion criteria were (a) known disease status (PD or SD assessed by Response Evaluation Criteria in Solid Tumors [RECIST] version 1.0 criteria [22]) according to computed tomography (CT) scan or magnetic resonance imaging (MRI) and (b) 18F‐FDG PET and CT scans performed within a 30‐day interval (indication to perform 18F‐FDG PET was given at each center based on specific clinical scenarios). All patients with familial syndromes (multiple endocrine neoplasia type I, von Hippel‐Lindau) were excluded. Data were prospectively collected according to a shared protocol on NEN patients’ management and thus retrospectively analyzed.
Patients in whom the primary tumor site was unknown were also included if they were believed to belong to the small bowel due to the presence of a carcinoid syndrome and reliable histological and immunohistochemical criteria, after other common primary sites had been ruled out by conventional imaging procedures (CT or MRI, as appropriate, and 68Ga PET TC), as previously reported by other authors [23].
The diagnosis of EP NENs was based on conventional histological findings [24]. All cases were classified according to the World Health Organization 2010 classification [25] and were staged using the TNM staging system [26], [27]. Follow‐up was performed by both participating centers according to the ENETS standard of care [28] by CT or MRI every 3–6 months depending on the clinical scenario. In addition, assessment of somatostatin receptors expression was performed in all patients, as suggested by ENETS guidelines [29], by 68Ga PET CT examination. Disease status (PD or SD) at the time of study entry was assessed by comparing CT scan performed at the time of 18F‐FDG PET examination and previous CT or MRI performed within the previous 6 months. At the time of study entry, as well as during subsequent follow‐ups, the disease status was assessed according to RECIST version 1.0 criteria [22]. The research protocol was approved by the local ethics committee, and full informed consent was obtained from all patients.
Imaging Protocols
All examinations were carried out by highly experienced nuclear medicine physicians at each center. 18F‐FDG PET CT studies were performed on hybrid PET/CT systems (Philips Medical Systems, Cleveland, OH [at the Sant'Andrea Hospital Rome Center] and Siemens mCT Biograph, Germany [at the San Raffaele Milan‐Negrar Hospital Center]) after patients received an adequate dose of FDG (2.96 mBq/kg and 3.7 mBq/kg at the Rome and Milan‐Negrar Centers, respectively).
In all cases, patients fasted for at least 6 hours before starting the study; blood glucose level was measured before injection and was less than 160 mg/dL in all patients. PET images were recorded for 3 minutes per bed position, from head to midthigh, 1 hour after intravenous injection. Low‐dose CT scans for attenuation correction and anatomic location were performed using a continuous spiral technique on a 64‐slice helical CT scanner with the following parameters: 120–140 kV, 90–100 mA, 0.5–0.8‐second tube rotation, 5 mm thickness. After acquisition attenuation, corrected PET images were fused with CT images and displayed in maximum intensity projections along the axial, sagittal, and coronal orthogonal planes.
Imaging Analysis
18F‐FDG PET images were reviewed by nuclear medicine physicians, well experienced in the field of NENs, who were unaware of the patients’ clinical data. The reviewers were asked to classify the scans as positive or negative after visual and qualitative evaluation. Each examination was considered to be positive when a focal uptake was higher than the liver radioactivity and negative in the absence of high‐uptake foci. The sites of known physiologic uptake, that is, kidney, ureter, bladder, and muscle‐skeletal symmetrical uptake, were not mentioned to avoid misinterpretations. The maximum standardized uptake value (SUVmax), a parameter used to achieve a semiquantitative evaluation of the 18F‐FDG PET uptake, was calculated, but no quantitative or semiquantitative parameters were considered for the reviewers’ qualitative assessment.
Data Analysis
The diagnostic ability of 18F‐FDG PET to correctly identify disease status was evaluated by assessing the proportion of cases in which it correctly identified the disease status (positive 18F‐FDG PET in patients with documented PD, negative 18F‐FDG PET in patients with documented SD). A 2 × 2 table was used to assess 18F‐FDG PET sensitivity and specificity.
Progression‐free survival (PFS) was defined as the interval between radiological examinations and time of PD or patient death, if it occurred before documented PD. PFS, as well as overall survival (OS) analyses, were performed using the Kaplan‐Meier method, and the results were compared by using the log‐rank test. Multiple regression was used to determine the association between selected clinical and imaging variables and the presence of documented PD at the time of study entry, which was considered as a dependent variable. The analysis of risk factors for prediction of PD during follow‐up was performed by univariate and multivariate analysis using a Cox proportional hazard model. Risk factors were expressed as hazard ratio (HR). The multivariate model was constructed by the “enter” method, after including all variables that had resulted significant at the univariate analysis. The distribution of continuous variables was reported as median and interquartile range [IQR; 25th to 75th percentiles]. Comparison between subgroups was performed by Fisher exact test or chi‐square test, as appropriate. All p values <.05 were considered significant. The statistical analysis was performed using a dedicated software (MedCalc16.4.3; MedCalc Software, Ostend, Belgium, www.medcalc.be).
Results
Included Patients
Of 142 patients with advanced EP NENs initially reviewed for potential eligibility, 93 (median age 60 years, IQR 50–68 years), with a median overall follow‐up of 39 months (IQR 15–50 months) were included in the final analysis. The remaining 49 patients were excluded because of the following reasons: 18F‐FDG PET and CT scan were performed with a time frame >1 month (n = 34); follow‐up data were not available (n = 13); Ki67 value was unknown (n = 2).
The median interval between patients’ initial diagnosis with EP NENs and enrollment into the study was 13 months (IQR 6–85 months). Patients’ general features are summarized in Table 1.
Table 1. General features of the 93 evaluated patients.
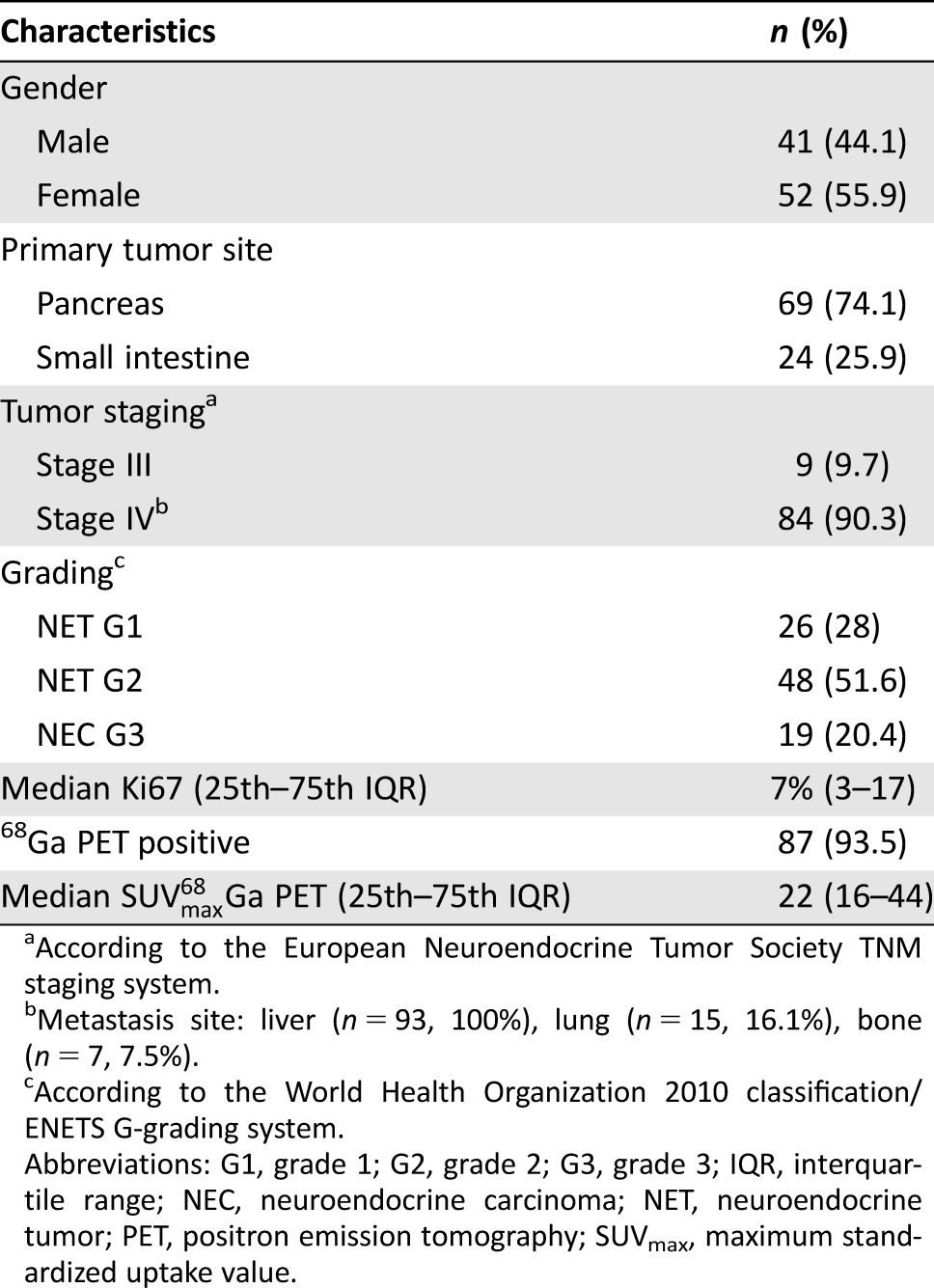
According to the European Neuroendocrine Tumor Society TNM staging system.
Metastasis site: liver (n = 93, 100%), lung (n = 15, 16.1%), bone (n = 7, 7.5%).
According to the World Health Organization 2010 classification/ENETS G‐grading system.
Abbreviations: G1, grade 1; G2, grade 2; G3, grade 3; IQR, interquartile range; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor; PET, positron emission tomography; SUVmax, maximum standardized uptake value.
The most frequent medical treatments that patients had received before the study entry were somatostatin analogues, 69 patients (74.1%); everolimus, 18 patients (19.3%); peptide receptor radionuclide therapy (PRRT), 18 patients (19.3%); and systemic chemotherapies, 6 patients (6.5% [capecitabine and temozolomide, 4 patients; cisplatin and etoposide, 1 patient; capecitabine and oxaliplatin, 1 patient]). A total of 8 patients (8.6%) had undergone liver disease cytoreduction (surgical debulking, 4 patients; chemoembolization, 4 patients), and the primary tumor had been previously resected in 43 patients (46.2%). A total of five patients (5.4%) did not receive any treatments before entering the study due to a recent diagnosis; however, the disease status was also known in these patients given the availability of previous radiological examination to assess the tumor behavior.
According to RECIST 1.0 criteria, 64 patients (68.8%) had PD, as documented by radiological imaging procedures, whereas the remaining 29 patients (31.2%) had SD at the time of study enrollment.
18F‐FDG PET Results and Association with Disease Status at Study Entry
A total of 62 patients (66.7%) had a positive 18F‐FDG PET, median SUVmax being 6 (IQR 4–8.2), whereas 18F‐FDG PET was negative in the remaining 31 patients (33.3%). As far as the primary tumor site was concerned, 18F‐FDG PET was positive in 47 patients with pancreatic NENs (68.1%) and in 15 patients (62.5%) with intestinal NENs (p = .449).
Disease status was correctly identified by 18F‐FDG PET in 83 of 93 patients, thus resulting in a diagnostic accuracy of 89.2% (Table 2). The sensitivity and specificity of 18F‐FDG PET to identify the disease status correctly were 90.6% and 86.2%, respectively.
Table 2. 18F‐FDG‐PET diagnostic accuracy.
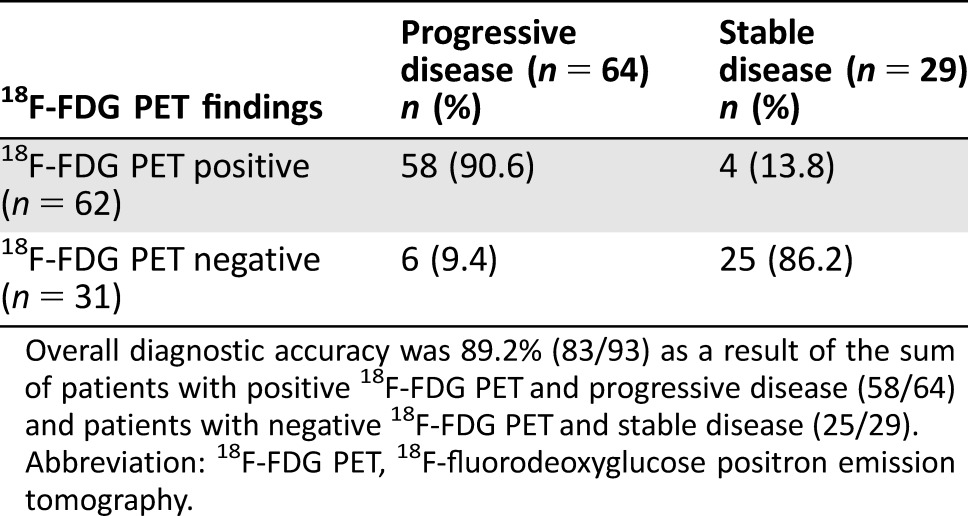
Overall diagnostic accuracy was 89.2% (83/93) as a result of the sum of patients with positive 18F‐FDG PET and progressive disease (58/64) and patients with negative 18F‐FDG PET and stable disease (25/29).
Abbreviation: 18F‐FDG PET, 18F‐fluorodeoxyglucose positron emission tomography.
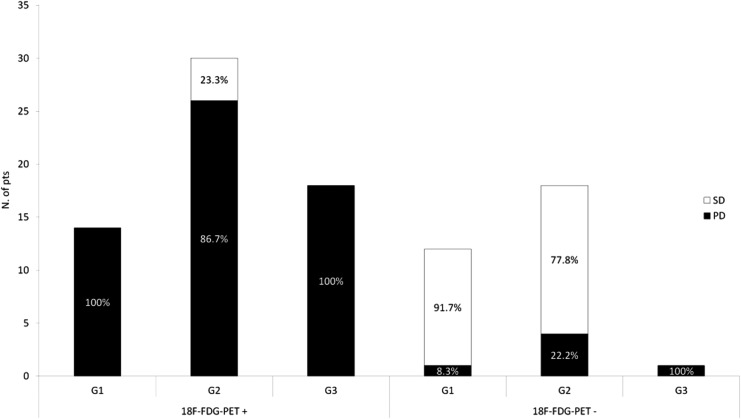
As far as the relationship between 18F‐FDG PET findings and tumor grading was concerned, a statistically significant higher proportion of 18F‐FDG PET‐positive examinations were observed in patients with higher tumor grade (p = .01; Table 3; Fig. 1).
Table 3. 18F‐FDG PET findings according to G grading categories.
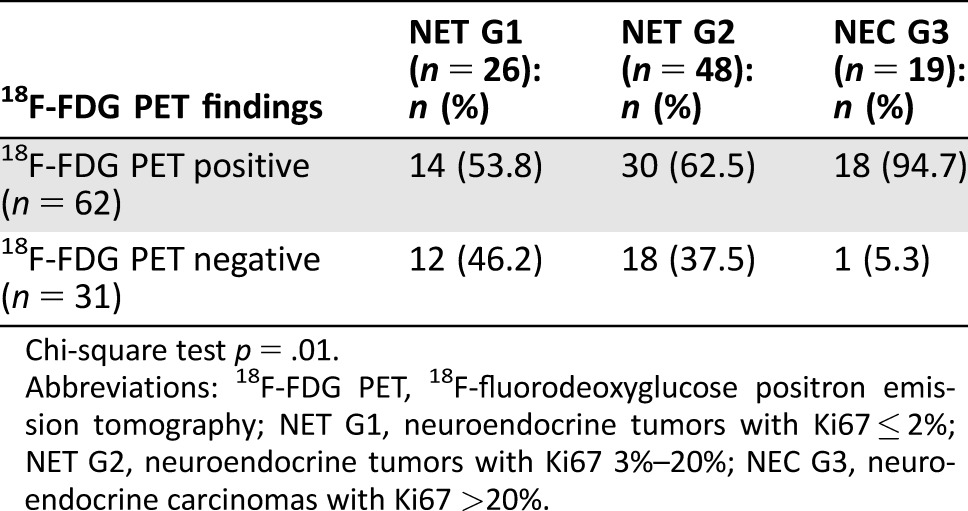
Chi‐square test p = .01.
Abbreviations: 18F‐FDG PET, 18F‐fluorodeoxyglucose positron emission tomography; NET G1, neuroendocrine tumors with Ki67 ≤ 2%; NET G2, neuroendocrine tumors with Ki67 3%–20%; NEC G3, neuroendocrine carcinomas with Ki67 >20%.
Figure 1.
Relationship between 18F‐FDG PET findings and disease status at time of diagnosis, according to the G grading system.
Abbreviations: 18F‐FDG PET+, 18F‐fluorodeoxyglucose positron emission tomography positive; 18F‐FDG PET−, 18F‐fluorodeoxyglucose positron emission tomography negative; G1, grade 1 (Ki67 ≤ 2%); G2, grade 2 (Ki67 = 3%–20%); G3, grade 3 (Ki67 > 20%); PD, progressive disease; SD, stable disease.
A positive 18F‐FDG PET was significantly associated with PD at the time of study entry, as confirmed by univariate and multivariate regression analysis (Table 4), thus confirming the high ability of 18F‐FDG PET to predict tumor aggressiveness in these patients.
Table 4. Variables associated with progressive disease at the time of study entry.
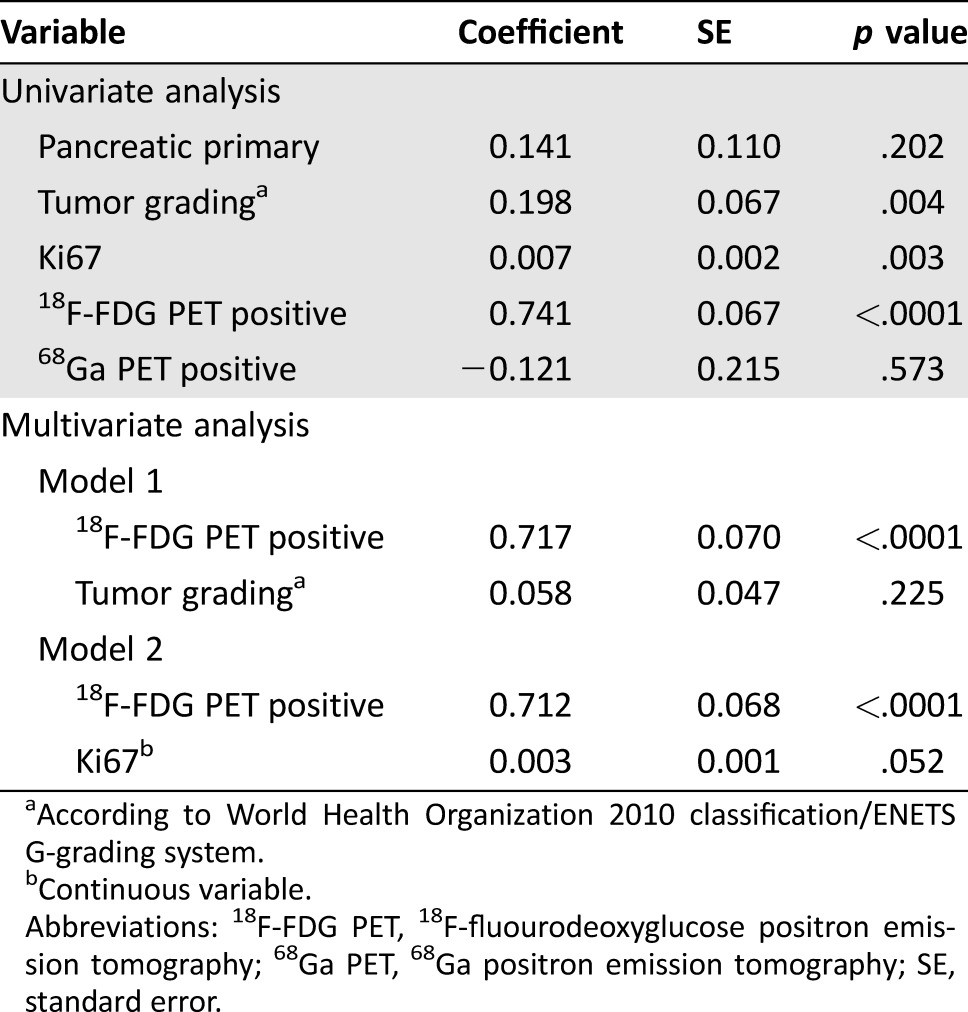
According to World Health Organization 2010 classification/ENETS G‐grading system.
Continuous variable.
Abbreviations: 18F‐FDG PET, 18F‐fluourodeoxyglucose positron emission tomography; 68Ga PET, 68Ga positron emission tomography; SE, standard error.
18F‐FDG PET Results and Association with Clinical Outcome
After the 18F‐FDG PET examination, patients received the following therapies during a median follow‐up time of 25 months (IQR 15–36): somatostatin analogues, 70 patients (75.2%); everolimus, 35 patients (37.6%); PRRT, 31 patients (33.3%); systemic chemotherapies, 12 patients (12.9% [capecitabine and temozolomide, 11 patients; cisplatin and etoposide, 1 patient]); and sunitinib, 5 patients (5.3%).
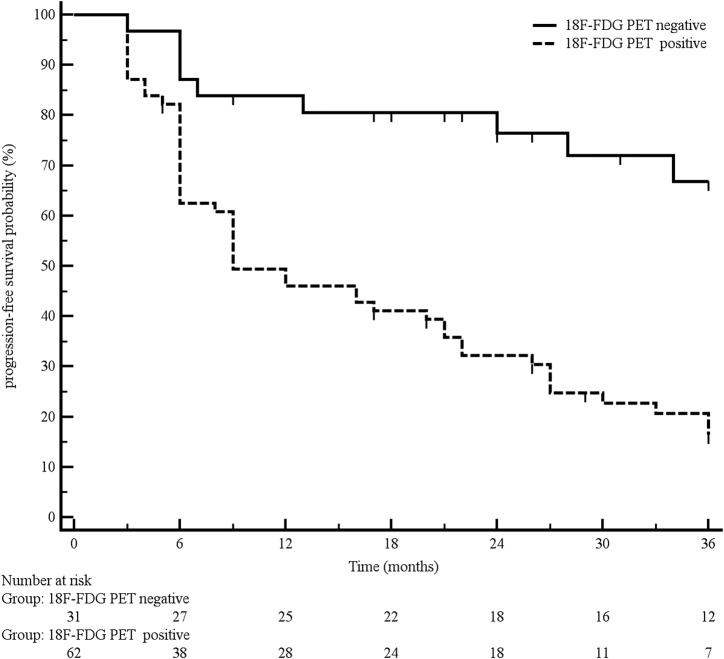
Overall, median PFS was 22 months. PFS was significantly longer in patients with a negative 18F‐FDG PET in comparison with 18F‐FDG PET‐positive ones, the median PFS being 50 and 9 months, respectively (p < .0001; Fig. 2).
Figure 2.
Progression‐free survival according to 18F‐FDG PET findings.
Abbreviation: 18F‐FDG PET, 18F‐fluorodeoxyglucose positron emission tomography.
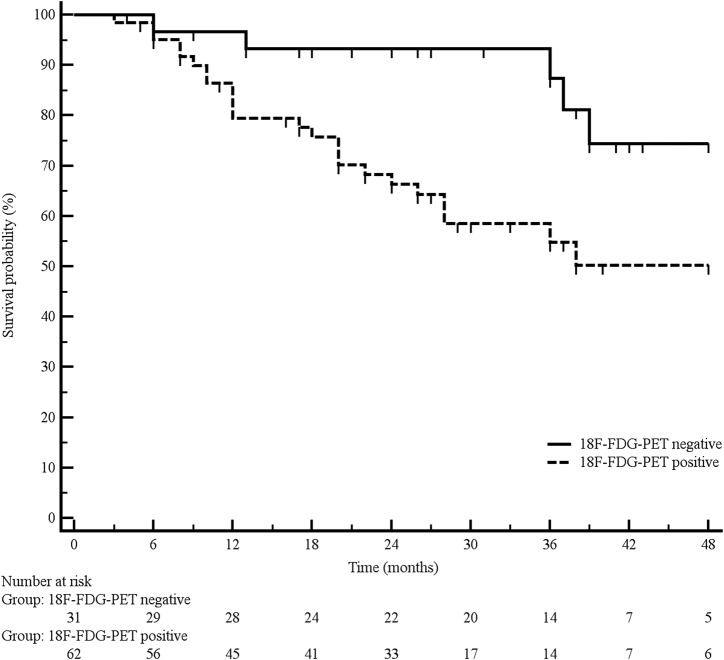
A total of 30 patients died during follow‐up, resulting in a mortality rate of 32.2%. Median OS was 60 months. A significantly longer OS was observed in 18F‐FDG PET‐negative patients in comparison with 18F‐FDG PET‐positive patients, median OS being not reached and 60 months, respectively (p = .008; Fig. 3).
Figure 3.
Overall survival according to 18F‐FDG PET findings.
Abbreviation: 18F‐FDG PET, 18F‐fluorodeoxyglucose positron emission tomography.
At univariate analysis, the predictors for increased risk of death during follow‐up were Ki67 (HR 1.02 for each increasing unit) and 18F‐FDG PET positivity (HR 3.19; p = .001 and p = .013, respectively). Both variables were also confirmed to be independent predictors for poor clinical outcome by multivariate analysis (p = .009 and p = .037, respectively).
Discussion
The possible clinical usefulness of 18F‐FDG PET in EP NENs has been extensively investigated over the last few years. Although the overall 18F‐FDG PET utility in aggressive NENs has already been investigated by other studies, conflicting results have been reported, possibly due to the heterogeneity of the enrolled populations in most of those studies, which have rarely focused on the relationship between 18F‐FDG PET findings and tumor behavior at the time of the examination.
Ezziddin et al. [30] investigated the predictive ability of 18F‐FDG PET on PFS and OS, suggesting that patients should be stratified according to the metabolic expression into three risk categories based on the glycolytic activity. In that study, the metabolic grade inversely correlated with survival stratification, thus proving to be a strong independent predictor for poor clinical outcome. However, the possible correlation with histological grading was not evaluated. Furthermore, the methodology proposed to identify the risk categories has not been validated so far.
A proposal to correlate 18F‐FDG PET findings with the WHO classification was made by Binderup et al. [18], who observed, over a relatively short follow‐up period (11.5 months), that 18F‐FDG PET SUVmax correlated with patients’ risk of death. An increased incidence of 18F‐FDG PET positivity was reported in tumors with increased Ki67, suggesting a possible correlation between the two parameters. However, that study was performed in a heterogeneous population of patients (digestive and bronchial NENs). Furthermore, the Ki67 proliferative index was not available for all patients, and the 18F‐FDG PET expression did not correlate with the disease status (PD or SD).
Similar findings were also reported by other studies [19], [20], which generally showed that 18F‐FDG PET positivity correlated with poor survival in NENs. Again, although the messages from these studies agree with that reported by the present work, they were usually performed in small, heterogeneous series of patients, including those with NENs arising both from the digestive system and from the lung.
In the present paper, a larger and homogeneous series of patients with advanced EP NENs with known disease status and Ki67 at the time of study enrollment was investigated in order to assess the relationship between 18F‐FDG PET findings and disease status and to try to better place this examination in the diagnostic algorithm of these patients.
As already mentioned, in the present study, disease status (SD or PD) was assessed according to RECIST version 1.0 criteria, which suggest evaluating tumor response basing on conventional CT or MRI techniques [22]. However, additional useful information on disease status during follow‐up might be provided by using 68Ga PET CT, given its ability to detect new metastatic lesions in progressive tumors [31].
The recent updated European Neuroendocrine Tumors Society guidelines, indeed, do not recommend the use of 18F‐FDG PET in EP NENs unless a grade 3 grading is present [32], [33]. This recommendation might be challenged by some of our findings.
In the present study, the association between 18F‐FDG PET positivity and the presence of PD was significantly stronger, compared with that of the G grading system or Ki67 analyzed as continuous variables, as confirmed by the multiple regression models summarized in Table 4. This figure highlights the role of this examination as a noninvasive, accurate tool able to identify unfavorable disease behavior that might be an alternative to Ki67, which, as is well known, is considered to be the strongest prognostic factor for these patients, as reported in both pancreatic and intestinal NENs [4], [5], [6], [7], [8], [34], [35].
Interestingly, a consistent proportion of patients with grade 1 neuroendocrine tumors (NETs) (53.8%) also had positive 18F‐FDG PET, and 37.5% of patients with grade 2 NETs had negative 18F‐FDG PET (Fig. 1). This finding suggests that 18F‐FDG PET findings do not depend on G grading alone, because it may also be positive in tumors with low proliferative activity. On the contrary, it significantly correlated with the disease behavior at the time of examination, again suggesting consideration of tumor behavior instead of Ki67 as the major factor influencing 18F‐FDG PET findings.
Guidelines from both the National Comprehensive Cancer Network and the European Neuroendocrine Tumor Society [36], [37] propose to consider a “watch and wait” strategy in the therapeutic algorithm of nonfunctioning, low‐grade NENs with limited tumor burden and known stable disease. However, if disease behavior is unknown (i.e., in newly diagnosed patients), positive 18F‐FDG PET may be helpful to identify those patients with significant risk of progression, avoiding unsafe observation before beginning antitumor therapy.
Conclusion
Although the present study is affected by some limitations, which, however, may be considered intrinsic to most studies investigating EP NENs (i.e., retrospective data analysis and heterogeneous, nonstandardized, therapeutic approaches received by the patients), we believe that some useful clinical messages might be drawn: (a) Because 18F‐FDG PET findings strongly correlate with disease behavior, they may provide useful information to better select patients with more aggressive disease. (b) In patients with unknown disease status, 18F‐FDG PET is able to provide relevant clinical information, suggesting the choice of a more aggressive therapeutic approach in patients with positive examination. (c) On the contrary, if the disease status (PD or SD) is already known, as confirmed by comparing cross‐sectional radiological examinations performed during previous follow‐up, 18F‐FDG PET might be avoided, because it would not give any additional information on tumor behavior. These findings may help physicians dealing with advanced EP NENs to better select patients for whom 18F‐FDG PET is really needed, in order to plan tailored therapeutic approaches in patients with high risk of predictable unfavorable clinical outcome.
Acknowledgments
This work was supported by the Italian Association for Neuroendocrine Tumors (www.ita-net.org).
Author Contributions
Conception/design: Maria Rinzivillo, Massimo Falconi, Gianfranco Delle Fave, Francesco Panzuto
Provision of study material or patients: Daniela Prosperi, Patrizia Pizzichini, Elsa Iannicelli, Matteo Salgarello
Collection and/or assembly of data: Maria Rinzivillo, Stefano Partelli, Gabriele Capurso, Elettra Merola, Francesca Muffatti
Data analysis and interpretation: Maria Rinzivillo, Francesco Scopinaro, Orazio Schillaci, Massimo Falconi, Gianfranco Delle Fave, Francesco Panzuto
Manuscript writing: Maria Rinzivillo, Francesco Panzuto
Final approval of manuscript: Maria Rinzivillo, Stefano Partelli, Daniela Prosperi, Gabriele Capurso, Patrizia Pizzichini, Elsa Iannicelli, Elettra Merola, Francesca Muffatti, Francesco Scopinaro, Orazio Schillaci, Matteo Salgarello, Massimo Falconi, Gianfranco Delle Fave, Francesco Panzuto
Disclosures
The authors indicated no financial relationships.
References
- 1. Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perren A, Couvelard A, Scoazec JY et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pathology, diagnosis and prognostic stratification. Neuroendocrinology 2017;105:196–200. [DOI] [PubMed] [Google Scholar]
- 3. Panzuto F, Merola E, Pavel ME et al. Stage IV gastro‐entero‐pancreatic neuroendocrine neoplasms: A risk score to predict clinical outcome. The Oncologist 2017;22:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rindi G, Falconi M, Klersy C et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J Natl Cancer Inst 2012;104:764–777. [DOI] [PubMed] [Google Scholar]
- 5. Pape UF, Jann H, Müller‐Nordhorn J et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008;113:256–265. [DOI] [PubMed] [Google Scholar]
- 6. Panzuto F, Nasoni S, Falconi M et al. Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 7. Panzuto F, Boninsegna L, Fazio N et al. Metastatic and locally advanced pancreatic endocrine carcinomas: Analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372–2377. [DOI] [PubMed] [Google Scholar]
- 8. Panzuto F, Campana D, Fazio N et al. Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology 2012;96:32–40. [DOI] [PubMed] [Google Scholar]
- 9. Vesselle H, Schmidt RA, Pugsley JM et al. Lung cancer proliferation correlates with [F‐18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 2000;6:3837–3844. [PubMed] [Google Scholar]
- 10. Pieterman RM, van Putten JW, Meuzelaar JJ et al. Preoperative staging of non‐small‐cell lung cancer with positron‐emission tomography. N Engl J Med 2000;343:254–261. [DOI] [PubMed] [Google Scholar]
- 11. Hutchings M, Loft A, Hansen M et al. FDG‐PET after two cycles of chemotherapy predicts treatment failure and progression‐free survival in Hodgkin lymphoma. Blood 2006;107:52–59. [DOI] [PubMed] [Google Scholar]
- 12. Gallamini A, Hutchings M, Rigacci L et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: A report from a joint Italian‐Danish study. J Clin Oncol 2007;25:3746–3752. [DOI] [PubMed] [Google Scholar]
- 13. Blay JY, Bonvalot S, Casali P et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:66–78. [DOI] [PubMed] [Google Scholar]
- 14. Wiering B, Krabbe PF, Jager GJ et al. The impact of fluor‐18‐deoxyglucose‐positron emission tomography in the management of colorectal liver metastases. Cancer 2005;104:2658–2670. [DOI] [PubMed] [Google Scholar]
- 15. Pasquali C, Rubello D, Sperti C et al. Neuroendocrine tumor imaging: Can 18F‐fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg 1998;22:588–592. [DOI] [PubMed] [Google Scholar]
- 16. Kayani I, Bomanji JB, Groves A et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga‐DOTATATE (DOTA‐DPhe1,Tyr3‐octreotate) and 18F‐FDG. Cancer 2008;112:2447–2455. [DOI] [PubMed] [Google Scholar]
- 17. Partelli S, Rinzivillo M, Maurizi A et al. The role of combined Ga‐DOTANOC and (18)FDG PET/CT in the management of patients with pancreatic neuroendocrine tumors. Neuroendocrinology 2014;100:293–299. [DOI] [PubMed] [Google Scholar]
- 18. Binderup T, Knigge U, Loft A et al.18F‐fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;6:978–985. [DOI] [PubMed] [Google Scholar]
- 19. Garin E, Le Jeune F, Devillers A et al. Predictive value of 18F‐FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med 2009;50:858–864. [DOI] [PubMed] [Google Scholar]
- 20. Bahri H, Laurence L, Edeline J et al. High prognostic value of 18F‐FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: A long‐term evaluation. J Nucl Med 2014;55:1786–1790. [DOI] [PubMed] [Google Scholar]
- 21. Cingarlini S, Ortolani S, Salgarello M et al. Role of combined 68Ga‐DOTATOC and 18F‐FDG positron emission tomography/computed tomography in the diagnostic workup of pancreas neuroendocrine tumors: Implications for managing surgical decisions. Pancreas 2017;46:42–47. [DOI] [PubMed] [Google Scholar]
- 22. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 23. Arnold R, Rinke A, Klose KJ et al. Octreotide versus octreotide plus interferon‐alpha in endocrine gastroenteropancreatic tumors: A randomized trial. Clin Gastroenterol Hepatol 2005;3:761–771. [DOI] [PubMed] [Google Scholar]
- 24. Rindi G, Bordi C, La Rosa S et al. Gastroenteropancreatic (neuro)endocrine neoplasms: The histology report. Dig Liver Dis 2011;43(suppl4):S356–S360. [DOI] [PubMed] [Google Scholar]
- 25. Bosman FT, Carneiro F, Hruban RH et al., eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: IARC Press, 2010.
- 26. Rindi G, Klöppel G, Couvelard A et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch 2007;451:757–762. [DOI] [PubMed] [Google Scholar]
- 27. Rindi G, Klöppel G, Alhman H et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch 2006;449:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plöckinger U, Wiedenmann B, de Herder WW. ENETS consensus guidelines for the standard of care in neuroendocrine tumors. Neuroendocrinology 2009;90:159–161. [DOI] [PubMed] [Google Scholar]
- 29. Kwekkeboom DJ, Krenning EP, Scheidhauer K et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Somatostatin receptor imaging with (111)in‐pentetreotide. Neuroendocrinology 2009;90:184–189. [DOI] [PubMed] [Google Scholar]
- 30. Ezziddin S, Adler L, Sabet A et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F‐FDG PET: Feasibility of a metabolic grading system. J Nucl Med 2014;55:1260–1266. [DOI] [PubMed] [Google Scholar]
- 31. Merola E, Pavel ME, Panzuto F et al. Functional imaging in the follow‐up of enteropancreatic neuroendocrine tumors: Clinical usefulness and indications. J Clin Endocrinol Metab 2017;102:1486–1494. [DOI] [PubMed] [Google Scholar]
- 32. Niederle B, Pape UF, Costa F et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 2016;103:125–138. [DOI] [PubMed] [Google Scholar]
- 33. Falconi M, Eriksson B, Kaltsas G et al. ENETS consensus guidelines update for the management of patients with functional pancreatic Neuroendocrine tumors and non‐functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grozinsky‐Glasberg S, Mazeh H, Gross DJ. Clinical features of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci 2015;22:578–585. [DOI] [PubMed] [Google Scholar]
- 35. Kaltenborn A, Matzke S, Kleine M et al. Prediction of survival and tumor recurrence in patients undergoing surgery for pancreatic neuroendocrine neoplasms. J Surg Oncol 2016;113:194–202. [DOI] [PubMed] [Google Scholar]
- 36. Pavel M, O'Toole D, Costa F et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (nen) and nen of unknown primary site. Neuroendocrinology 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network . Clinical practice guidelines in oncology: Neuroendocrine tumors. Version 2. 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed June 10, 2017.