FIG 2.
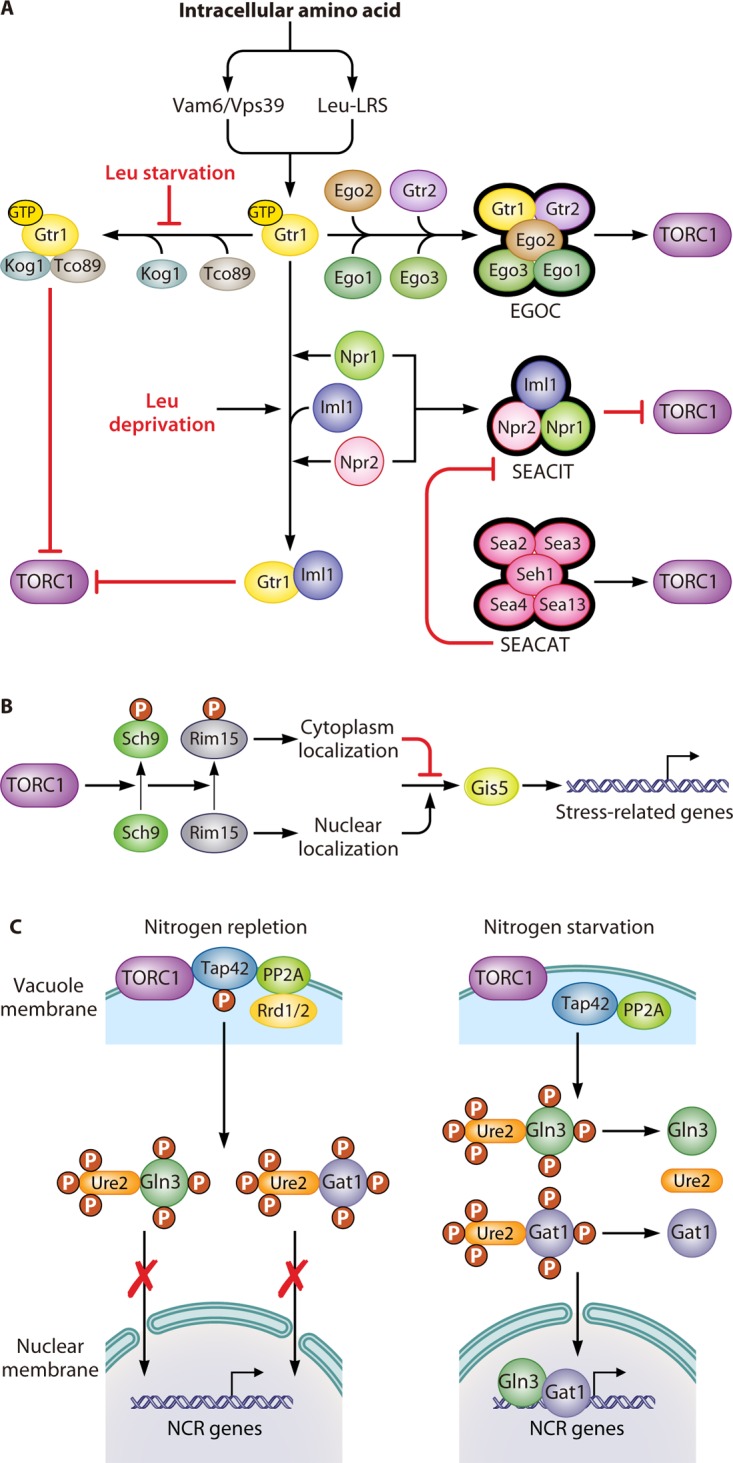
Regulation of the TOR pathway. (A) Upstream of the TOR pathway. The guanine nucleotide-binding status of Gtr1 plays an essential role in the sensing of intracellular amino acids by the TOR pathway. When sufficient levels of amino acids are present in the cytoplasm, this signal transduces through two factors, Vam6/Vps39 and LRS. They induce Gtr1 to recruit four other proteins, Gtr2, Ego1, Ego2, and Ego3, which comprise the EGOC, which activates the TORC1 complex. The GTP-bound format of Gtr1 can physically interact with Tco89 and Kog1, resulting in the inhibition of the TORC1 complex. However, this inhibition can be prevented by leucine starvation. Additionally, the SEACIT, which consists of Iml1, Npr2, and Npr3, inhibits TORC1 activity through an Npr2- and Npr3-dependent transient interaction between Iml1 and Gtr1. Leucine deprivation can promote this kind of transient interaction. Furthermore, the SEACAT activates the TORC1 complex by inhibiting the activity of the SEACIT. (B) Sch9, a downstream effector of the TOR pathway, controls the expression of stress-related genes. The activation of TORC1 promotes the phosphorylation of Sch9, which results in the subsequent phosphorylation of Rim15. Phosphorylated Rim15 is sequestrated in the cytosol, where it prevents Gis1 from activating stress-related genes. (C) The Tap42-PPase complex, another downstream effector of the TOR pathway, controls the expression of NCR-related genes. The activation of the TORC1 complex mediates the phosphorylation of Tap42 under nitrogen repletion conditions. Phosphorylated Tap42 binds with PP2A, along with either one of the regulatory proteins Rrd1 or Rrd2, and localizes on the vacuolar membrane, which facilitates their interaction with the TORC1 complex. The sequestration of the Tap42-PP2A complex on the vacuolar membrane prevents them from dephosphorylating the transcriptional activators Gln3 and Gat1, which prevents them from leaving the cytoplasm. In contrast, the inhibition of the TORC1 complex by nitrogen starvation or rapamycin treatment promotes the dephosphorylation of Tap42 and the release of Tap42-PP2A from the vacuolar membrane. Free Tap42-PP2A leads to the dephosphorylation of Gln3 and Gat1, thereby facilitating their translocation into the nucleus, where they activate NCR genes.
