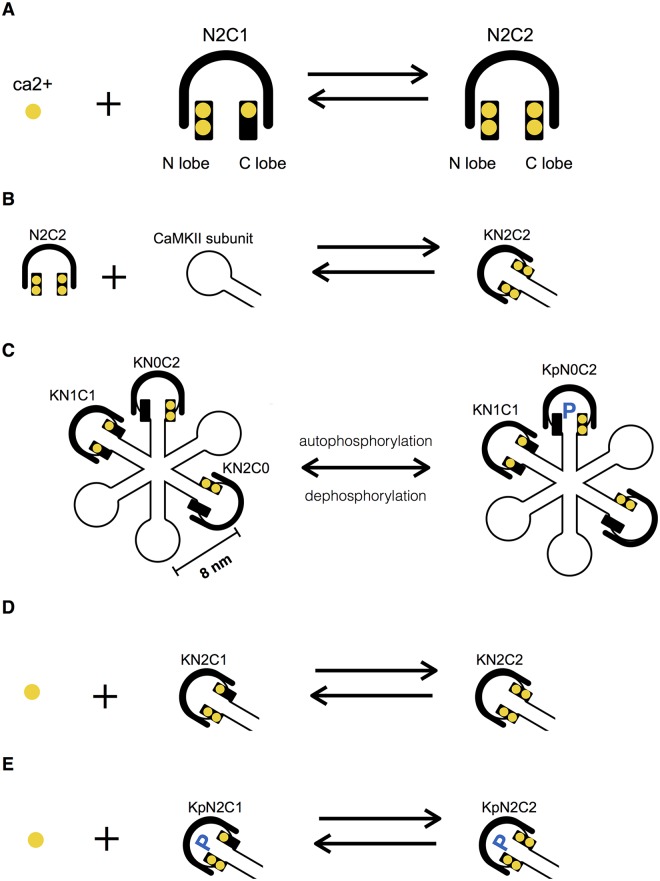
Fig 1. Types of Ca2+-CaM-CaMKII interactions modeled.
(A) Ca2+ binds to a CaM molecule that already has 3 Ca2+ bound, making the CaM fully loaded. Each CaM has two lobes, an N lobe and a C lobe. Each lobe has 2 Ca2+ binding sites. (B) A CaM molecule binds to a CaMKII subunit, activating the subunit. Each subunit of the CaMKII holoenzyme can interact with CaM independently. (C) CaMKII autophosphorylation is neighbor sensitive and follows one direction (arbitrarily chosen as left to right here). When both a subunit and its neighbor to the left are activated, the subunit can be phosphorylated. (D) CaM bound to a CaMKII subunit interacts with Ca2+ at an increased affinity. (E) CaM bound to a phosphorylated CaMKII subunit also interacts with Ca2+ at a further increased affinity. Notation: K represents an unphosphorylated CaMKII subunit; Kp is a phosphorylated subunit; NiCj is CaM with i Ca2+ ions bound at the N lobe and j Ca2+ ions bound at the C lobe.