Abstract
Background
Daytime sleepiness is recognized in childhood brain tumor survivors. Our objective was to determine prevalence, risk factors for PSG/MLST proven hypersomnia/narcolepsy, and response to stimulants in childhood brain tumor survivors.
Methods
Standard PSG/MSLT criteria were used to diagnose hypersomnia/narcolepsy. Medical records of brain tumor survivors having undergone a PSG/MSLT were reviewed for the diagnostic code of hypersomnia/narcolepsy. Survivors with hypersomnia/narcolepsy were matched with 2-3 survivors without reported hypersomnia/narcolepsy by age at tumor diagnosis, gender, and time from tumor diagnosis.
Results
Between January 2000 to April 2015, 39 of the 2336 brain tumor patients treated at our institution were diagnosed with hypersomnia/narcolepsy for a prevalence rate of 1670/100,000. Hypersomnia/narcolepsy was diagnosed at a median of 6.1 years (range 0.4 - 13.2) from tumor diagnosis and 4.7 years (range -1.5 - 10.4) from cranial radiation. Midline tumor location (OR 4.6, CI 1.7 - 12.2, p = 0.002) and anti-epilepsy drug (AED) use (OR 11, CI 2.4-54) correlated with hypersomnia/narcolepsy while radiation dose >30 Gray trended towards significance (OR 1.8, CI 0.9-3.6); posterior fossa tumor location reduced the risk (OR 0.1, CI 0.04 - 0.5, p=0.002). AED use also correlated with midline tumor location. Thirty-seven survivors were treated with stimulants and reported improved wakefulness and school performance (response rate CI 0.97 [0.86 - 0.99] and 0.83 [0.65 - 0.94]).
Conclusion
Prevalence of hypersomnia/narcolepsy among childhood brain tumor survivors was higher than the general population. Tumor location and radiation dose were possible risk factors, and stimulants were reported to be beneficial.
Keywords: Brain tumor, childhood, hypersomnia, narcolepsy
Introduction
The International Classification of Sleep Disorders, Third Edition, identifies narcolepsy, idiopathic hypersomnia, hypersomnia due to a medical disorder, or narcolepsy due to medical disorder as the most common central disorders that cause excessive daytime sleepiness [1]. Narcolepsy is further divided into two types, 1) with Orexin deficiency and presence of cataplexy and 2) without Orexin deficiency and absence of cataplexy. Diagnosis of narcolepsy requires a clinical diagnosis of excessive sleepiness, sleep latency ≤10 minutes, and ≥2 sleep onset rapid eye movement periods on multiple sleep latency test (MSLT). Hypersomnia has similar diagnostic criteria with a sleep latency ≤ 10 minutes and ≤1 sleep onset rapid eye movement periods on MSLT. Prevalence of these disorders is not clear but an estimated prevalence of narcolepsy in Western countries is 20-50/100,000 [2].
Many brain tumors arise from structures in proximity to the Orexin producing cells in the hypothalamus, which may be damaged during surgery and further compromised during focal or whole brain irradiation. Indeed pituitary/hypothalamic endocrine dysfunction is well described in childhood brain tumor survivors [3], and questionnaire based sleep studies of childhood brain tumor survivors suggest a higher prevalence of excessive daytime sleepiness [4–8]. A previous review identified 7 of 17 childhood brain tumor survivors with excessive daytime sleepiness who had an overnight polysomnogram (PSG) followed by MSLT as having narcolepsy or hypersomnia due to a medical condition [9].
Although clinical staff at our institution tries proactively to seek symptoms of excessive daytime sleepiness in childhood brain tumor patients and survivors, the magnitude of pathological sleepiness in brain tumor survivors is not known. The aim of this study was to identify brain tumor survivors with and without narcolepsy/ hypersomnia to determine the prevalence of narcolepsy/hypersomnia, to identify associated risk factors, and survivor/parent reported response of sleepiness to pharmacological therapy.
Methods
We identified 156 survivors from our hospital data base with the diagnoses of sleep disordered breathing, hypersomnia, and/or narcolepsy. Electronic medical records of each of these 156 were then reviewed after obtaining Institutional Review Board approval. Inclusion criteria for entry in to hypersomnia/narcolepsy group included: diagnosis of brain tumor; treatment at our institution; MSLT proven hypersomnia or narcolepsy; a negative PSG the night before for moderate to severe obstructive or central apnea; and absence of periodic leg movements associated frequent awakenings. Children on our active craniopharyngioma protocol were excluded as they are receiving sleep studies as part of the protocol. Results of PSG and/or MSLT were available for 63 survivors and 39 of these survivors were identified as having hypersomnia or narcolepsy based on their PSG/MSLT results. Reasons for exclusion in those that had PSG/MSLT included moderate to severe obstructive sleep apnea in 11, normal PSG/MSLT in 2, no MSLT in 2, and 9 were participating in craniopharyngioma protocol. Each survivor with hypersomnia/narcolepsy was matched to 2-3 (n=110) brain tumor survivors with no reported day time sleepiness (Figure 1). In addition, survivors were matched for age at tumor diagnosis, gender, and follow-up time since tumor diagnosis. Multiple variables were evaluated as potential risk factors for the development of hypersomnia/narcolepsy (Table 1). Electronic medical records were reviewed for medication history and response of hypersomnia/narcolepsy to pharmacologic therapy based on survivor or parent report. Response to therapy was defined as no response to therapy, partial response where there was improvement in daytime sleepiness but not complete resolution, and complete resolution. Improvement in academic performance was assessed by parent response to whether school grades have improved or not. Neuroimaging was reviewed by a single investigator (RBK) to confirm location of the tumor which was defined as: posterior fossa tumor; cortical, including subcortical area; midline tumors, including the parasellar, hypothalamus, optic pathway, pineal region and third ventricle; and paramedian tumors arising from the deep gray nuclei.
Figure 1.
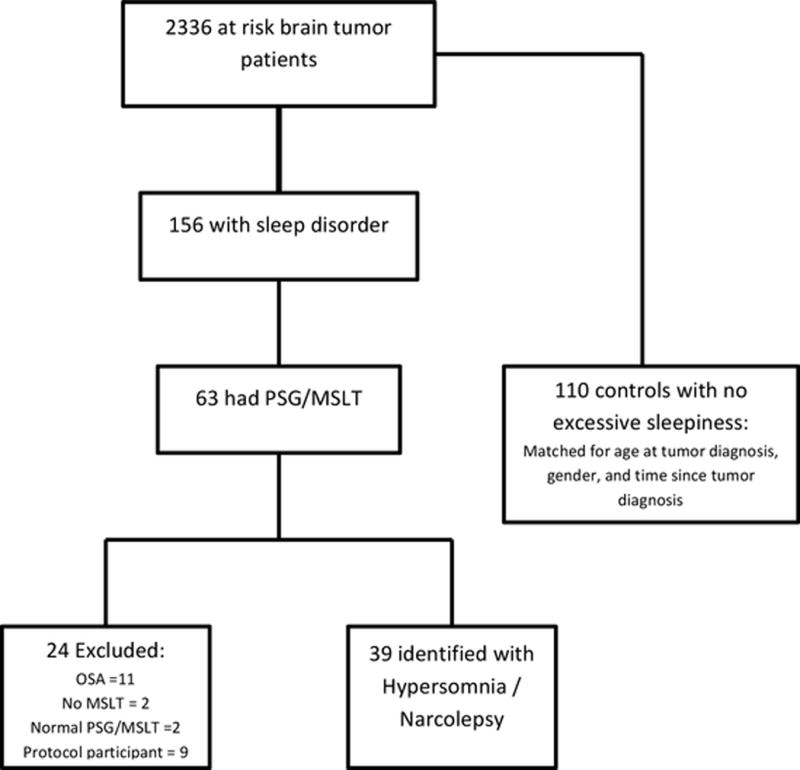
Flow diagram of study participant recruitment.
Table 1.
Demographic and descriptive statistics of study and control group.
| Variable | Study Group (n=39) |
Control Group (n=110) |
P-value |
|---|---|---|---|
|
| |||
| GenderA | NA | ||
| Male | 24 (62%) | 67 (61%) | |
| Female | 15 (38%) | 43 (39%) | |
|
| |||
| Median age at tumor diagnosisA | 10.1 years (range 0.8 – 17.7) |
10.1 years (range 0.4 – 18.6) |
NA |
|
| |||
| Tumor location | <0.001 | ||
| Cortical | 4 (10%) | 16 (15%) | |
| Midline | 26 (67%) | 30 (27%) | |
| Paramedian | 4 (10%) | 8 (7%) | |
| Posterior fossa | 5 (13%) | 56 (51%) | |
|
| |||
| Median follow-up since tumor diagnosisA | 10.2 years (range 2.4 to 18.2) |
9.8 years (range 1.7 to 18.4) |
0.9 |
|
| |||
| Median time from RT to hypersomniaB | 4.7 years (−1.46 – 10.4) |
NA | NA |
|
| |||
| Median time from tumor diagnosis to hypersomnia | 6.1 years (range 0.4 – 13.2) |
NA | NA |
|
| |||
| Number of surgeries | 0.6 | ||
| 0 | 4 (10) | 11 (10%) | |
| 1 | 29 (74%) | 74 (67%) | |
| 2 | 6 (15%) | 19 (17%) | |
| 3 | 0 (0%) | 6 (5%) | |
|
| |||
| Ventricular/cavity shunt | 0.3 | ||
| Yes | 12 (31%) | 24 (22%) | |
| No | 27 (69%) | 86 (78%) | |
|
| |||
| Radiation | 0.3 | ||
| Yes | 33 (85%) | 75 (68%) | |
| No | 6 (15%) | 35 (32%) | |
|
| |||
| Mean radiation dose | 45.9 (standard deviation 20.2) |
30.3 (standard deviation 23.3) |
<0.001 |
|
| |||
| Mean Body Mass Index | 25.4 (standard deviation 6.3) |
25.4 (standard deviation 9.4) |
0.6 |
|
| |||
| Diabetes Insipidus | 17 (44%) | 8 (7%) | <0.001 |
|
| |||
| Hypopituitarism | 29 (74%) | 50 (45%) | 0.003 |
|
| |||
| Anti-epilepsy drug | 18 (46%) | 10 (9%) | <0.001 |
matched prior to study; RT, radiation therapy;
in those that received RT
All survivors in the hypersomnia /narcolepsy group had a nocturnal PSG followed the next day by an MSLT in an American Academy of Sleep Medicine accredited laboratory. Standardized techniques were used to score sleep and awake cycles and trials. All studies were conducted by experienced technologists and interpreted by a physician board certified in sleep medicine (MSW).
Statistics
Descriptive statistics were used to summarize the demographic data (number and percent, mean and standard deviation, or median and range). Fisher’s exact tests and Wilcoxon rank sum tests with P<0.05 were used to compare the discrete and continuous variables between the case and control group respectively.
In addition, conditional logistic regression was used to evaluate those variables that were found significant on univariable analysis. Diabetes Insipidus and hypopituitarism were not tested in this model as polychoric correlation analysis revealed strong correlations between diabetes insipidus and hypopituitarism (0.99), and hypopituitarism with radiation (0.69), and >30 Gray radiation dose (0.89). In the model, the response variable was a binary variable of one for cases or zero for controls, and the predictors were tumor location and radiation. Each matched set was treated as from a stratum. Anti-epilepsy drug (AED) is used as a covariate to adjust for drug regimens. Radiation was also a one/zero binary variable indicating whether radiation was given or not. Tumor location was a categorical variable representing four categories of cortical, midline, paramedian, and posterior fossa. The posterior fossa category was used as the baseline category. Three dummy variables that represented the difference between the posterior fossa category and the other three categories, respectively, were included in a conditional logistic regression. The effect of radiation was estimated together with the three dummy variables. Moreover, we compared the effect of each tumor location compared to the average effect of the other locations. For each comparison, we created a dummy variable which was one for the category of interest and zero for the other categories. The conditional logistic regression using radiation and the dummy variable as predictors was refitted for each comparison.
For exploratory objectives, conditional logistic regression was used to study the correlations of hypersomnia with the other clinical variables, including radiation dose, number of surgery, ventricular or cavity shunt, AED use, and body mass index (BMI). The three dummy variables representing the tumor locations were also included into the model because they accounted for more variation and improved testing power for the other variables. A two-sided significance level of p<0.05 was used for all Wald tests which assessed each predictor/dummy variable in the models. False discovery rate correction was used to adjust for multiple comparisons.
To study the response of hypersomnia to pharmacotherapy, we focused only on the case group and calculated the response rate and its 95% confidence interval. The response rate was estimated by the proportion of patients for whom the pharmacologic treatment alleviated the daytime sleepiness. The exact confidence interval was calculated with Pearson-Klopper method. Statistical analyses were conducted using R Version 3.3.0.
Results
We identified 13 survivors fulfilling diagnostic criteria for hypersomnia due to medical disorder and 26 with narcolepsy due to medical disorder without cataplexy. A total of 2336 children with brain tumors were treated at our institution during the study period resulting in an approximate prevalence rate of 1670/100,000 for the hypersomnia/narcolepsy. Clinical and demographic variables of the study and control group are provided in Table 1 and tumor pathologies in Table 2. As anticipated because of matching, there was no difference between study and control group at age of tumor diagnosis and in median follow-up time since tumor diagnosis. Median time since radiation treatment was 4.7 years (-1.5 – 10.4 years) in the study group and 9.3 years (range 2.1 – 17.3 years) in the control group for those that were treated with radiation. Tumor grade were not compared because of relatively small number of varying pathologies.
Table 2.
Pathology and grade of tumors
| Pathology | Case (n = 39) |
Control (n = 110) |
|---|---|---|
| Astrocytoma Grade I and II | 10 | 42 |
| Astrocytoma Grade III and IV | 0 | 2 |
| Ependymoma | 4 | 10 |
| Craniopharyngioma | 16 | 13 |
| Germ cell | 3 | 6 |
| Medulloblastoma / PNET | 2 | 28 |
| ATRT | 0 | 4 |
| Pineal tumor | 0 | 2 |
| Miscellaneous | 4 | 3 |
Overnight PSG was abnormal in 11 of the 39 survivors with mild obstructive sleep apnea present in 7; periodic leg movement sleep in 5; and one survivor with both periodic leg movements and mild obstructive sleep apnea. Eighteen study survivors were taking at least one anti-seizure drug for seizures or headaches at the time of their sleep evaluation and 14 were on an anti-depressant for anxiety or headaches. Five survivors with hypersomnia/narcolepsy due to medical condition were on low dose benzodiazepine for anxiety and none were on anti-psychotics. Additionally, 10 survivors were on as needed non-sedating anti-histamine drugs. The majority of the survivors with hypersomnia/narcolepsy (n=36) were under the care of an endocrinologist and most of these were receiving multiple hormone replacements. Six survivors experienced tumor relapse at a median of 5 years (range 0.1 to 11.2 years) prior to onset of daytime excessive sleepiness and two developed progressive disease 2.2 and 4.2 years after the onset of hypersomnia symptoms.
The study and control group were found to have differences in tumor location on regression analysis (Table 3). Midline tumor location strongly correlated with the presence of hypersomnia/narcolepsy (adjusted odds ratio [OR] 4.6, CI 1.7 to 12.2, p = 0.002). Use of AED maintained association with hypersomnia / narcolepsy (OR 11, CI 2.4-54, p= 0.002). However, use of AED also strongly correlated with midline tumor location (OR 3.2, p = 0.009). Radiation dose of >30 Gray to brain trended towards significance with the presence of hypersomnia/narcolepsy (adjusted OR 1.8, CI 0.9 to 3.6, p=0.08), while posterior fossa tumor location negatively correlated with the presence of hypersomnia/narcolepsy (adjusted OR 0.1, CI 0.04 to 0.5, p = 0.002). The percentage of patients that received cranial radiation, number of surgeries, presence of a shunt, and high BMI were not found to be associated with the presence of hypersomnia/narcolepsy.
Table 3.
Study variables and their correlation with hypersomnia/narcolepsy
| Variable | Odds Ratio (95% Confidence Interval) |
P-value |
|---|---|---|
|
| ||
| Location | ||
| Midline vs Others | 5.3 (2.2 – 12.7) | <0.001 |
| Paramedian vs Others | 1.8 (0.5 – 7.0) | 0.4 |
| Cortical vs Others | 0.9 (0.3 – 3.0) | 0.9 |
| Posterior fossa vs Others | 0.1 (0.03 – 0.4) | <0.001 |
|
| ||
| Radiation Therapy | 2.7 (0.9 – 8.9) | 0.09 |
|
| ||
| >30 Gray Radiation dose | 2.2 (1.2 – 3.9) | 0.01 |
|
| ||
| Number of surgeries | 0.7 (0.4 – 1.1) | 0.1 |
|
| ||
| Ventricular or cavity shunt | 1.6 (0.6 – 4.5) | 0.4 |
|
| ||
| Body Mass Index | 0.7 (0.4 – 1.5) | 0.5 |
Pharmacologic therapy was prescribed to 37 survivors with documented hypersomnia/narcolepsy and included modafinil, armodafinil, methylphenidate, amphetamine/dextroamphetamine, and atomoxetine. The most common medications prescribed were modafinil and methylphenidate. As reported by survivors and parents, response to treatment in the study group could be ascertained based on clinical interview in all except one treated with stimulants. Nineteen of the 36 (53%) reported complete resolution of daytime sleepiness, 16 (44%) reported partial improvement, and 1 (3%) reported only mild to no improvement; response rate CI 0.97 (0.86 – 0.99). First line medication was not well tolerated or effective in 8 (22%) and symptoms improved on an alternative stimulant. Effect of pharmacotherapy on school grades could not be determined in 9, improved in 25 and was reported as unchanged in 5; response rate 0.83 (0.65 – 0.94).
Discussion
The prevalence rate of 1670/100,000 for hypersomnia/narcolepsy in our cohort of childhood brain tumor survivors is much higher than a prevalence of 20-50/100,000 reported in the general population [2]. We believe this may be an underestimate of true prevalence in childhood brain tumor survivors as many with mild to moderate symptoms may not have been referred for a consultation with a sleep specialist or PSG, and more specifically evaluation with an MSLT. Other concurrent symptoms such as fatigue may be attributed to tumor and its treatment without consideration of excessive daytime sleepiness, hypersomnia, and narcolepsy. It is known that adult childhood cancer survivors subjectively report higher prevalence of fatigue and daytime sleepiness with 67% of survivors endorsing a clinical history of fatigue in one study [6]. Consequently, adult childhood cancer survivors who report fatigue and sleep disturbance have been found to have increased risk for cognitive impairment [8]. Physicians, survivors, and parents may focus on fatigue (rather than sleepiness) as an explanation for the complaint of excessive daytime sleepiness, thus delaying the sleep evaluation and the potential diagnosis of hypersomnia/narcolepsy.
In addition to the clinical complaint of fatigue and sleepiness, tumor location and radiation dose may predict hypersomnia/narcolepsy. Our findings demonstrated that midline tumors and radiation dose >30 Gray were associated with hypersomnia and narcolepsy. Association with midline tumors is not a novel finding and has been previously suggested in multiple studies.10–13 Some of these included PSG / MSLT evaluations and reported presence of narcolepsy without cataplexy as well. Our study is unique in having a control group and suggests a role of radiation dose as well. A prior questionnaire based study also suggested role of cranial radiation with subjective sleep disturbance [7]. A more recent prospective study of children with craniopharyngioma also reported high incidence of central sleep disorder based on PSG, MSLT, and clinical assessment [10]. Some of these studies also suggested high BMI as a risk factor for sleep disturbance. We could not confirm an association between BMI and hypersomnia/narcolepsy. This is likely related to the difference in methodology as one study was questionnaire based,6 and in the other only 17 of 31 survivors underwent a MSLT [9]. Furthermore, we excluded survivors that had moderate to severe obstructive sleep apnea, a population where elevated BMI may be more relevant.
Association of anti-epilepsy drug use with hypersomnia / narcolepsy is difficult to explain. Headache was the reason for their use in about half of those taking the anti-epilepsy drugs. We did find strong correlation between the use of these drugs and midline tumor location and this may explain this unexpected association with sleep disorder. We are unaware of any reported association between narcolepsy and anti-epilepsy drugs. Only a small proportion of those with hypersomnia / narcolepsy had tumor progression prior to the development of hypersomnia and many of these received radiation therapy at relapse. Only a larger and prospective study will be able to define roles of surgical injury, tumor related destruction of Orexin cells, and radiation effect in causation of hypersomnia.
Presence of hypopituitarism and diabetes insipidus both correlated with presence of hypersomnia or narcolepsy in a univariable analysis. However, our data could not confirm this association on multivariable analysis as diabetes insipidus and hypopituitarism had very strong correlation with each other and hypopituitarism with radiation treatment. Underlying unconfirmed hypothesis regarding etiology of hypersomnia involves orexin deficiency, a hormone produced by the hypothalamic neurons. It may be very difficult to prove hypopituitarism as an independent predictor as it is uncommon in non-irradiated brains.
The treatment plan for hypersomnia/narcolepsy should be developed on an individual basis after discussion with the patient and parent/caregiver. Strategic naps are rarely practical or efficacious enough when used alone, and we found napping alone to be effective in only two survivors. First line pharmacological intervention includes modafinil or armodafinil; however, these drugs do not have FDA approval for use in patients less than 18 years of age. For patients with concurrent attention deficit disorder and/or processing speed impairment, stimulants may be more suitable. Medications are slowly titrated over a number of weeks with monitoring for adverse events. Prescribed medications are typically well tolerated and discontinuation due to adverse effects is uncommon. Our review found brain tumor survivors with hypersomnia/narcolepsy responded well to pharmacological intervention with improved wakefulness and school performance as per survivor and parent report.
As is inherent with retrospective studies, our review has limitations. Response to treatment was ascertained by survivor or parent report and academic performance was not verified. No validated questionnaire was used to assess improvement in wakefulness, alertness, or maintenance of wakefulness. It is also likely that we have underestimated the true prevalence of hypersomnia/narcolepsy in childhood brain survivors, as not all brain tumor survivors are followed by a neurological service where a sleep history is proactively obtained. Additionally, we also excluded survivors that are currently enrolled in our craniopharyngioma trial where sleep evaluation is being routinely obtained; preliminary analysis suggested a high prevalence of sleep disorder in this population [10]. It is also possible that some of the survivors in the control group may have hypersomnia/narcolepsy, at least of mild to moderate degree, which may not have been reported to physicians by parents.
In conclusion, prevalence of hypersomnia/narcolepsy in childhood brain tumor survivors is likely much higher than in the general population. Higher dose of cranial radiation and midline tumor location increase hypersomnia/narcolepsy risk, while posterior fossa tumor location may be protective against development of hypersomnia/narcolepsy. Pharmacologic therapy is generally well tolerated, improves symptoms, and likely also improves school performance. Future prospective evaluation in clinical trials will need to confirm our findings and help establish the true prevalence of hypersomnia and narcolepsy in childhood brain tumor survivors, as well as the response to therapy. Clinicians caring for brain tumor survivors with symptoms of excessive daytime sleepiness and/or fatigue should evaluate these complaints with an overnight PSG and an MSLT which are imperative for a definitive diagnosis of hypersomnia/narcolepsy.
Acknowledgments
Funding: This study was supported by Cancer Center Support Grant (CA21765) from the National Cancer Institute; and by the ALSAC.
References
- 1.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Chemaitilly W, Li Z, Huang S, Ness KK, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brimeyer C, Adams L, Zhu L, et al. Sleep complaints in survivors of pediatric brain tumors. Support Care Cancer. 2016;24:23–31. doi: 10.1007/s00520-015-2713-x. [DOI] [PubMed] [Google Scholar]
- 5.Verberne LM, Maurice-Stam H, Grootenhuis MA, Van Santen HM, Schouten-Van Meeteren AY. Sleep disorders in children after treatment for a CNS tumour. J Sleep Res. 2012;21:461–469. doi: 10.1111/j.1365-2869.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS) Sleep. 2008;31:271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan VG, Gapstur R, Gross CR, et al. Sleep disturbances in adult survivors of childhood brain tumors. Qual Life Res. 2013;22:781–789. doi: 10.1007/s11136-012-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117:2559–2568. doi: 10.1002/cncr.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrell BN, Wise M, Schoumacher RA, et al. Excessive daytime sleepiness and sleep-disordered breathing disturbances in survivors of childhood central nervous system tumors. Pediatr Blood Cancer. 2012;58:746–751. doi: 10.1002/pbc.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacola LM, Conklin HM, Scoggins MA, et al. Investigating the Role of Hypothalamic Tumor Involvement in Sleep and Cognitive Outcomes Among Children Treated for Craniopharyngioma. J Pediatr Psychol. 2016;41:610–622. doi: 10.1093/jpepsy/jsw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller HL, Müller-Stöver S, Gebhardt U, Kolb R, Sörensen N, Handwerker G. Secondary narcolepsy may be a causative factor of increased daytime sleepiness in obese childhood craniopharyngioma patients. J Pediatr Endocrinol Metab Suppl. 2006;1:423–429. doi: 10.1055/s-2006-974095. [DOI] [PubMed] [Google Scholar]
- 12.Rosen GM, Bendel AE, Neglia JP, Moertel CL, Mahowald M. Sleep in children with neoplasms of the central nervous system: case review of 14 children. Pediatrics. 2003;112:46–54. doi: 10.1542/peds.112.1.e46. [DOI] [PubMed] [Google Scholar]
- 13.Rosen G, Brand SR. Sleep in children with cancer: case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. 2011;19:985–994. doi: 10.1007/s00520-010-0921-y. [DOI] [PubMed] [Google Scholar]


