Figure 37.6.
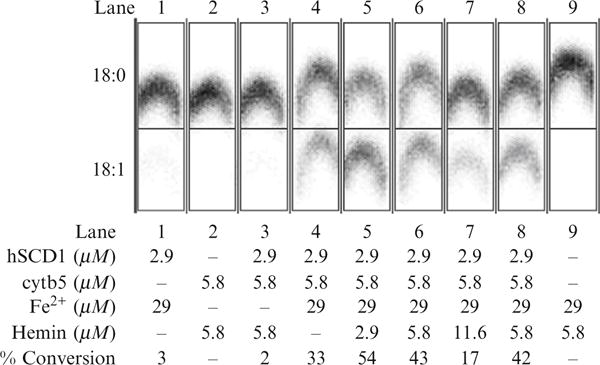
Catalytic activity observed from various reconstitutions of the complex of human stearoyl-CoA desaturase and human cytochrome b5 produced by cell-free translation and purified by density gradient centrifugation. The soluble domain of human cytochrome b5 reductase was expressed in E. coli and added to these assays. The influence of adding Fe2+ (required for hSCD1 activity) and hemin (required for cytb5 activity) is also demonstrated. Lane 1, hSCD1 plus iron. Lane 2, cytb5 plus hemin. Lane 3, cotranslation of hSCD1 and cytb5 supplemented with hemin. Lane 4, cotranslation of hSCD1 and cytb5 supplemented with Fe2+. Lane 5, cotranslation of hSCD1 and cytb5 supplemented with Fe2+ and 0.5 equiv. of hemin. Lane 6, cotranslation of hSCD1 and cytb5 supplemented with Fe2+ and 1 equiv. of hemin. Lane 7, cotranslation of hSCD1 and cytb5 supplemented with Fe2+ and 2 equiv. of hemin. Lane 8, mixture of separately translated and purified hSCD1 with separately translated and purified cytb5 supplemented with Fe2+ and 1 equiv. of hemin. Lane 5, cotranslation of hSCD1 and cytb5 supplemented with Fe2+ and 0.5 equiv. of hemin. Adapted from Goren and Fox (2008).
