Abstract
Acute respiratory distress syndrome (ARDS) is a common and devastating disorder. Alcohol use disorders (AUDs) increase ARDS risk and worsen outcomes through mechanisms that may include enhancement of pulmonary oxidative stress. Alcohol consumption increases activity of the enzyme xanthine oxidoreductase (XOR) that contributes to production of both reactive oxygen species (ROS) and uric acid, a damage-associated molecular pattern. These by-products have the potential to modulate proinflammatory pathways, such as those involving cyclooxygenase (COX)-2, and to activate the nucleotide-binding domain, leucine-rich-containing family, pyrin-domain containing-3 (NLRP3) inflammasome. We sought to determine if pulmonary and systemic XOR activity was altered by AUDs. Bronchoscopy with bronchoalveolar lavage (BAL) and blood sampling was performed in otherwise healthy human subjects with AUDs and controls. Uric acid in epithelial-lining fluid, derived from BAL, was substantially higher among individuals with AUDs and did not normalize after 7 days of abstinence; serum uric acid did not differ across groups. XOR enzyme activity in fresh BAL cells and serum was significantly increased in subjects with AUDs. XOR protein in BAL cells from AUD subjects was increased in parallel with COX-2 expression, and furthermore, mRNA expression of NLRP3 inflammasome components was sustained in LPS-stimulated BAL cells from AUD subjects in conjunction with increased IL-1β. Our data suggest that AUDs augment pulmonary and systemic XOR activity that may contribute to ROS and uric acid generation, promoting inflammation. Further investigations will be necessary to determine if XOR inhibition can mitigate alcohol-associated pulmonary oxidative stress, diminish inflammation, and improve ARDS outcomes.
Keywords: inflammasome, cyclooxygenase-2, bronchoscopy, human
INTRODUCTION
The acute respiratory distress syndrome (ARDS) is a devastating disorder, affecting ~190,000 people annually in the United States. In-hospital mortality in ARDS remains unacceptably high, at 27–45% (9, 35, 36, 45, 48, 66), and is responsible for 75,000 deaths and 3.6 million hospital days annually (53). Results from multisite clinical trials have led to better ARDS survival and less morbidity, including decreased time on mechanical ventilation (9a, 62). However, in the past decade, despite additional multicenter clinical trials, outcomes in ARDS have failed to improve further (50, 51, 60). One reason that improvement in outcomes has stalled may be related to the heterogeneous nature of ARDS in terms of its underlying biology and severity of illness (46).
Notably, people with alcohol use disorders (AUDs), including alcohol abuse and alcohol dependence, are at increased risk for ARDS development (37, 39) and for poorer outcomes, including mortality and prolonged hospitalization (13, 39). As such, on the ARDS spectrum, the phenotype of AUD-associated ARDS is more severe. In data examined from 1,037 patients enrolled between 2007 and 2011 in randomized clinical trials of ARDS therapies, 19% met criteria for severe alcohol misuse, defined by validated AUDs Identification Test (AUDIT) (4) scores, highlighting the prevalence of AUDs in ARDS. In this cohort, the odds ratio for the combined outcome of mortality or persistent hospitalization at 90 days was significantly higher among patients with severe alcohol misuse (odds ratio 1.70, 95% confidence interval 1.00–2.87, P = 0.048) and affected ~30% of patients (13). Reasons underlying the association between AUD and ARDS susceptibility with poor outcomes remain unclear, but augmented pulmonary oxidative stress related to alcohol consumption has been proposed as a mechanism. Our group has previously reported basally enhanced pulmonary oxidative stress in otherwise healthy subjects with AUDs (38, 65) that does not normalize after 7 days’ abstinence from alcohol (10, 11). Heightened pulmonary oxidative stress epitomizes ARDS (33, 59), where it is associated with deleterious effects on the viability and function of alveolar cells. It therefore appears plausible that AUDs prime the lung for subsequent injury.
One potential contributor to alcohol-associated oxidative stress is enhanced activity of the enzyme xanthine oxidoreductase (XOR). XOR is a molybdoflavin enzyme responsible for the terminal degradation of purines (i.e., adenine and guanine nucleotides) to uric acid, while generating reactive oxygen species (ROS), such as H2O2 and superoxide (29). XOR protein and activity are widely distributed across cells (30, 41, 54), including alveolar macrophages (AMs), cells responsible for driving the innate immune response and repair in ARDS (17, 63). Metabolism of ingested alcohol results in adenine nucleotide generation from rapid ATP consumption; additionally, NADH: NAD ratios are increased as alcohol is converted to acetate (64). Both favor increased XOR activity.
Human ARDS studies involving a relatively small number of patients have confirmed elevations in purine degradation products, both systemically and in the lung, consistent with heightened XOR activity (32, 47, 55). A number of investigations in diverse lung-injury models, which include hypoxia -induced, bleomycin-induced, cytokine-induced, and mechanical stress-related lung injury, have demonstrated upregulated pulmonary XOR activity in conjunction with increased lung and bronchoalveolar lavage (BAL) uric acid (1, 17, 21, 28, 31, 63). Pulmonary uric acid elevation in animal models has been associated with increased pulmonary inflammation, including production of the proinflammatory cytokines IL-1β and IL-18. Mechanisms implicated include activation of the nucleotide-binding domain, leucine-rich-containing family, pyrin-domain containing-3 (NLRP3) inflammasome (17, 31). The NLRP3 inflammasome is important in regulating IL-1β production in response to pathogen-associated molecular patterns, such as LPS, and damage-associated molecular patterns, including uric acid. IL-1β and IL-18 require caspase-1 (CASP1) activity to assume their mature forms; the NLRP3 inflammasome complex, in association with the sensor protein apoptosis-associated speck-like protein with a CASP recruitment domain (PYCARD or ASC), is responsible for activation of CASP1. Moreover, through ROS generation, XOR has been shown to regulate the proinflammatory mediator cyclooxygenase (COX)-2 (16, 43), whose metabolites are involved in inflammation, and can also activate NF-κB (52). Additionally, through effects on hypoxia-inducible factor 1α, XOR activity may further influence AM polarization, altering these cells’ response to infection (19).
Despite preclinical and clinical observations, the relationship between XOR activity and alcohol-associated ARDS remains poorly defined. XOR is unique among ROS-generating enzymes, in that specific, well-tolerated U.S. Food and Drug Administration-approved XOR inhibitors exist (6, 7). Therefore, the delineation of the impact of AUDs on XOR activity in the lung and systemically has clinical relevance, given the potential for XOR inhibitor administration to mitigate the development of, and poorer outcomes in, alcohol-associated ARDS. We have previously reported an association between AUDs and a proinflammatory AM phenotype, characterized by increased IL-1β and other proinflammatory cytokine production that may be mediated by oxidative stress (18, 42). We wished to examine further the relationship of AUDs to XOR activity, given this enzyme’s potential contribution to oxidative stress and uric acid production that may, in turn, drive inflammation. XOR enzyme activity has not been specifically assessed in the AUD setting, either in blood or in cells from BAL that are predominantly (>90%) AMs. Moreover, although elevated serum uric acid has been reported in AUDs (34), the uric acid in the epithelial lining fluid (ELF) of the lung has not been assessed specifically. We hypothesized that ELF uric acid would be greater among otherwise healthy individuals with AUDs. Furthermore, we postulated that AUDs would be associated with enhanced XOR activity both in BAL cells and systemically in serum.
MATERIALS AND METHODS
Subject Screening, Recruitment, and Enrollment
Subjects with AUDs were recruited between 2011 and 2015 at the Denver Comprehensive Addictions Rehabilitation and Evaluation Services Center, an inpatient detoxification facility affiliated with Denver Health and Hospital Administration (Denver, CO). Control subjects without AUDs were recruited via approved flyers and web advertisements in the Denver metropolitan area. Investigators submitted the protocols for the study to the Colorado Multiple Institutional Review Board, which approved this study, and all subjects provided written, informed consent before participation.
Subjects with AUDs were eligible to participate if they met all of the following criteria at study entry: 1) an AUDIT score of greater than or equal to eight for men or greater than or equal to five for women, 2) alcohol use within the 7 days before enrollment, and 3) age of ≥21. The AUDIT questionnaire is a standardized survey to detect current and previous alcohol abuse that has been validated in a variety of clinical settings (49). To meet eligibility as a control, control subjects’ AUDIT values were required to be less than eight for men or less than five for women. The screening of potential controls focused on balancing these individuals with AUD subjects in terms of age and smoking history. Exclusion criteria were chosen in an effort to minimize potential confounding related to medical comorbidities and included serious medical conditions requiring specialty care or prescription medication, as we have previously described (18). Furthermore, an abnormal chest radiograph or spirometry also led to exclusion, as did concurrent illicit drug use and abnormal nutritional status. Potential subjects >55 yr of age were also excluded to minimize the presence of concomitant but asymptomatic comorbidities. The ultimate sample sizes of subjects and controls were chosen based on feasibility to recruit comparable numbers of AUD subjects and controls who could be matched on the basis of smoking and prior investigations by our group.
Clinical Protocol
Eligible subjects with AUDs and controls were admitted to the University of Colorado Hospital’s Clinical & Translational Research Centers for blood sampling and bronchoscopy. All bronchoscopy procedures were performed using telemetry monitoring and standard conscious sedation protocols, as previously described (24). The bronchoscope was wedged into a subsegment of either the right middle lobe or the lingula. Three to four 50 ml aliquots of sterile, room temperature 0.9% saline were sequentially instilled and recovered with gentle aspiration. The first aspirated aliquot was not used in experiments for this investigation. All subsequent aliquots were combined and used in experiments, as representative of the distal airspaces. BAL samples were transported to the laboratory in sterile, 50 ml conical tubes. Whole-blood samples were also collected before bronchoscopy. In a pre-defined subset of AUD subjects, a second bronchoscopy was performed in an identical fashion after 7 days of observed inpatient abstinence on the University of Colorado Hospital’s Clinical & Translational Research Center unit to determine the impact of abstinence on outcome variables.
Laboratory Processing and Assays
Experiments performed used both banked biological samples from an Institutional Review Board-approved biorepository (e.g., uric acid measures), along with prospectively collected fresh samples (e.g., XOR activity measures). Due to limitations in sample quantity, particularly for BAL samples, and the nature of certain assays requiring fresh samples, not all experiments described were performed in samples from every individual enrolled. However, all subjects and controls underwent identical procedures for recruitment and enrollment and standardized protocols for sample collection.
The total amount of saline aspirated during the BAL procedure and the total amount of saline instilled into the lung were used to calculate the percent yield [(quantity aspirated/quantity instilled)] × 100] for each procedure. BAL specimens were centrifuged immediately (900 g, 10 min) after collection to separate cells from BAL fluid. Cellular viability was determined via Trypan blue exclusion; cells from all subjects were estimated to exceed 95% viability. Cell counts were performed on BAL samples to determine total cell number. Cytospins of BAL cells were examined after staining to determine differential cell types from a minimum of 200 cells by an observer blinded to the subject history. Whole blood was also centrifuged immediately (900 g, 10 min) after collection to isolate serum for subsequent analyses.
Uric acid measures.
Uric acid was measured in BAL fluid and serum via the o-phenylenediamine/uricase enzymatic method, measuring fluorescent oxidation products (excitation/emission: 410/550).
XOR activity assays.
XOR activity was measured between 1 and 3 h postcollection in fresh, unfrozen BAL cells (1 × 106) and serum (100 μl). XOR activity was determined at 293 nm by quantitating oxypurinol-inhibitable uric acid synthesis by spectrophotometry (Beckman Coulter, Brea, CA), adjusted for total protein (15, 16, 19). XOR activity was based on the enzyme kinetic activity for the duration of 12 min in the presence of the enzyme substrate xanthine and addition of NAD+ when the total XOR dehydrogenase activity was measured. Each sample (BAL cells and serum) was assessed in triplicate, and the average of these values is presented.
Immunostaining of BAL cells.
XOR protein was assessed on cytospins of BAL cells that had been prepared from BAL shortly after bronchoscopy, fixed in methanol, and stored in PBS at 4°C. Slides were rehydrated, and antigen retrieval was conducted with Vector antigen unmasking solution (H-3300; Vector, Burlingame, CA). Slides were then permeabilized with 0.2% Triton X-100 for 5 min and blocked with 10% donkey serum in PBS. Primary antibody solutions were as follows: 1:100 rabbit anti-XOR (ab6194; Abcam, Cambridge, MA) and rabbit anti-Id1 (sc-488; Santa Cruz Biotechnology, Dallas, TX). The immunostain was visualized with a donkey anti-rabbit IgG conjugated to DyLight 549 (711-506-152; Jackson ImmunoResearch, West Grove, PA). Slides were mounted with ProLong Gold (P36930; Molecular Probes, Eugene, OR), and images were captured in the Advanced Light Microscopy Core Facility at the University of Colorado Anschutz Medical Center Campus, using the Olympus IX81 inverted microscope, 100 W Hg lamp, and ORCA IIER charge-coupled device camera (Hamamatsu, Hamamatsu City, Japan), using SlideBook acquisition software (Intelligent Imaging Innovations, Denver, CO).
SDS-PAGE and Western immunoblot analysis of XOR protein in BAL cells.
Most reagents, buffers, substrates, and PAGE supplies were purchased from Sigma-Aldrich (St. Louis, MO). Media for cell culture was obtained from Thermo Fisher Scientific (Waltham, MA). FBS was obtained from Gemini Bio-Products (West Sacramento, CA). The following rabbit primary antibodies from Santa Cruz Biotechnology were used: anti NF-κB-p65 (sc-372), anti-COX-2 (sc-1747), anti-macrophage 2 (Mac-2; sc-32790), anti-nitric oxide synthase 2 (C11; sc-7271), and anti-arginase-1 (Arg-1; C2; sc-166920). Rabbit anti-β-actin (A2066) was purchased from Sigma-Aldrich. Rabbit antibody to XOR (ab6194) was purchased from Abcam. Anti-phospho-NF-κB p65 (Ser 468) antibody was purchased from Cell Signaling Technology (Danvers, MA). Goat anti-rabbit IgG (sc-2004; Santa Cruz Biotechnologies) horseradish peroxidase-conjugated secondary antibodies were used in the immunoblots. Western immunoblot analysis was performed, as described previously (15, 16). Briefly, isolated BAL cells were lysed in 0.1% Triton X-100 buffer. Protein concentrations were determined using the bicinchoninic acid assay (B9643; Sigma-Aldrich), and antigen-antibody complexes were detected by reaction with an ECL Western blotting detection kit, according to the manufacturer’s instructions (RPN2106; GE Healthcare Life Sciences, Pittsburgh, PA). Each experiment was run in duplicate or triplicate, and representative immunoblots are shown.
Message expression of NLRP3 inflammasome with stimulated BAL cells by qRT-PCR.
Five × 105 BAL cells/well were plated in 2 ml Roswell Park Memorial Institute (RPMI) medium with antibiotics (10-040-CV; Corning, Corning, NY) in 12-well plates. Cells were cultured for 1 h (37°C, 10% CO2), and then medium was removed and immediately replaced with RPMI medium only or RPMI medium and 1 μg/ml LPS (from Escherichia coli: 055:B5; Sigma-Aldrich). Serum was not added to BAL culture medium in an effort to replicate more closely serum-free conditions in the lung. All wells were cultured for 18 h (37°C, 10% CO2); cell-culture supernatants and cells were collected separately and stored at −80°C. Gene expression for NLRP3, PYCARD or ASC, and CASP1 was assessed by quantitative RT-PCR (qRT-PCR). Cultured BAL cells were lysed using buffer RLT from the RNeasy Plus Mini Kit (74134; Qiagen, Valencia, CA) and then homogenized using QIAshredder columns (79654; Qiagen) and stored at −80°C. RNA was extracted using the RNeasy Plus Mini Kit, according to the manufacturer’s instructions. After elution, RNA quantity and quality were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific). For all available samples, 1 μg total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (4374966; Thermo Fisher Scientific). The manufacturer’s protocol was followed, and cDNA was quantified using a NanoDrop spectrophotometer. For the qRT-PCR, 100 ng cDNA was brought up to 9 μl with nuclease-free water and combined with 1 μl TaqMan Gene Expression Assay and 10 μl TaqMan Gene Expression Master Mix (4369016; Thermo Fisher Scientific). Assays (including a no-template control) were performed in triplicate under standard real-time PCR conditions (50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s, followed by 60°C for 1 min) using a real-time PCR system (model 7300; Thermo Fisher Scientific) with sequence-detection software. Inventoried TaqMan Gene Expression Assays (Thermo Fisher Scientific) were used to measure mRNA expression levels for NLRP3 (assay ID Hs00918082_m1), PYCARD (assay ID Hs01547324_gH), and CASP1 (assay ID Hs_00354836). TATA box-binding protein (assay ID Hs00427620_m1) was used as the endogenous control.
IL-1β measurements.
Cell-culture supernatants (from experiments described above) were thawed and used neat with the V-PLEX Human Proinflammatory Panel I 4-Plex (K15052D-1; Meso Scale Diagnostics, Rockville, MD), following the manufacturer’s protocol. Each sample was measured in duplicate.
Statistics
Comparisons of continuous data were conducted using Wilcoxon’s/Kruskal-Wallis (rank sums) nonparametric testing, with post hoc tests where applicable. When differences in paired data were examined, Wilcoxon’s signed-rank testing was used. Categorical data were compared across groups using Fisher’s exact test. Correlations in data were examined with Spearman’s test. The comparative threshold method was used to examine changes in relative gene expression of NLRP3, PYCARD, and CASP1 in cells pre- and post-LPS stimulation, stratified by AUD or control groups (56). P <0.05 was deemed to be significant. JMP Pro 13 (SAS Institute, Cary, NC) was used to conduct statistical analyses.
RESULTS
Demographics of Subjects Who Underwent BAL
For analyses of BAL uric acid, data and samples from otherwise healthy subjects with AUDs and controls, in four groups based on active smoking habits, were used (Table 1). Ages differed across the four groups (P = 0.006), driven by the younger age of the control nonsmokers; however, median ages across groups spanned 9 yr. Fewer women were in the control smoker group. Subjects with AUDs had substantially higher AUDIT scores than controls. Similar smoking habits were present among subjects with and without AUDs. Spirometry data differed across the four groups; however, median forced expiratory volume in first second of exhalation (FEV1)/forced vital capacity ratios were within 3%, and median FEV1 percent predicted values were within 8%. The yield of the BAL procedure was significantly higher in nonsmoking controls compared with the other three subgroups (P ≤ 0.003 in post hoc comparisons).
Table 1.
Alcohol use disorder subjects and controls with epithelial lining fluid uric acid measurements
| Control Nonsmokers | Control Smokers | AUD Nonsmokers | AUD Smokers | P | |
|---|---|---|---|---|---|
| n | 21 | 24 | 32 | 50 | |
| Age, yr | 38 (33–42) | 42 (38–49) | 44 (39–48) | 47 (40–49) | 0.006* |
| Sex (%men) | 14/21 (67%) | 10/24 (42%) | 25/32 (78%) | 44/50 (88%) | 0.0004 |
| AUDIT score | 2 (1–4) | 2 (1–3) | 29 (17–33) | 28 (21–33) | <0.0001 |
| Pack-year smoking | n/a | 17 (10–21) | n/a | 15 (4–20) | 0.52 |
| FEV1/FVC ratio, %predicted | 82 (79–85) | 80 (77–83) | 80 (75–82) | 79 (74–82) | 0.05 |
| FEV1, %predicted | 102 (96–110) | 93 (85–103) | 99 (89–108) | 95 (88–101) | 0.03 |
| Percent yield of BAL procedure | 59 (49–71) | 46 (36–54) | 44 (35–57) | 45 (33–53) | 0.0003* |
Values are medians (interquartile range). AUD, alcohol use disorder; AUDIT, AUD identification test; FEV1, forced expiratory volume in first second of exhalation; FVC, forced vital capacity; BAL = bronchoalveolar lavage; n/a, not applicable.
Control nonsmokers significantly (P < 0.05) less than the other 3 subject groups in post hoc analyses.
Uric Acid Measurements in ELF
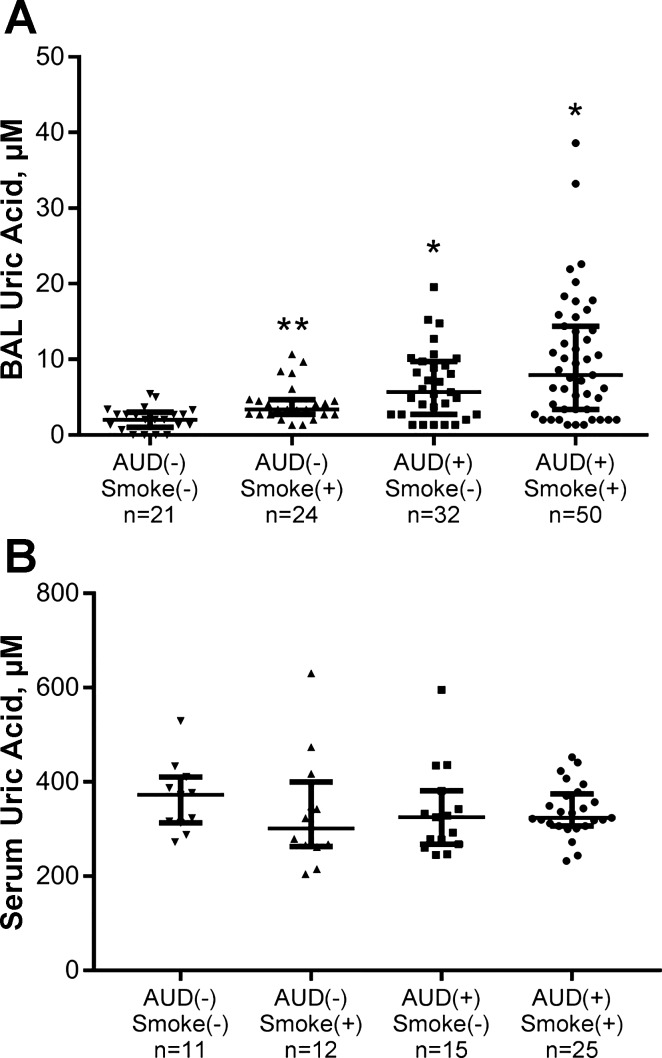
Uric acid was measured in ELF derived from BAL across the four subject types. Significant differences were observed across the four groups (P < 0.0001, by Wilcoxon’s/Kruskal-Wallis testing; Fig. 1A). In post hoc analyses to determine differences within groups, comparisons between AUD nonsmokers and control nonsmokers indicated increased ELF uric acid in the AUD group (P < 0.0001). Similarly, values among AUD smokers differed substantially from control smokers (P = 0.006). The comparison of control subject smokers with nonsmokers similarly indicated higher values among the smoking group (P = 0.001). Values between control smokers and AUD nonsmokers were not substantially different (P = 0.10). When ELF uric acid values were adjusted for percent yield of the BAL procedure, across-group differences persisted (P = 0.0008), with significantly higher values of ELF uric acid in AUD smokers compared with control smokers (P = 0.02) and AUD nonsmokers compared with control nonsmokers (P = 0.009) in post hoc analyses. Correlations between AUDIT scores and ELF uric acid were examined to establish if a potential dose response between alcohol consumption and ELF uric acid was present. AUDIT scores [range 0–39, median 18 with interquartile range (IQR) 3–30] were modestly associated with ELF uric acid (ρ = 0.43, P < 0.0001). Given the relationship that has been noted between smoking and serum uric acid values (22, 40) and the observation of higher ELF uric acid between control smokers and nonsmokers, we examined the correlation between smoking intensity and ELF uric acid. The examination of data between pack-year smoking and ELF uric acid values revealed a weak, positive correlation (ρ = 0.33, P = 0.0001). Values of ELF uric acid were not associated with age (P = 0.69), sex (P = 0.53), or spirometry (e.g., FEV1).
Fig. 1.
Uric acid was measured in either epithelial lining fluid [ELF; derived from bronchoalveolar lavage (BAL) fluid] or serum via the o-phenylenediamine/uricase enzymatic method. A: ELF uric acid was assessed in control subjects (both current smokers, n = 24, and nonsmokers, n = 21) and in subjects with alcohol use disorders (AUDs; both current smokers, n = 50, and nonsmokers, n = 32). Significant differences across the 4 groups were observed (P < 0.0001). In post hoc analyses, AUD subjects’ (both smokers and nonsmokers) uric acid values exceeded those in corresponding control smokers and nonsmokers (*P ≤ 0.006 for each comparison). Furthermore, ELF uric acid values were greater among smoking controls compared with nonsmokers (**P < 0.001) in post hoc testing. B: serum uric acid was measured in control subjects (both current smokers, n = 12, and nonsmokers, n = 11) and in subjects with AUDs (both current smokers, n = 25, and nonsmokers, n = 15). Values did not substantially differ among the 4 subject groups (P = 0.53). Medians with IQR are presented. Wilcoxon’s/Kruskal-Wallis testing was used for overall comparison, followed by individual Wilcoxon’s tests to examine differences between each pair.
Uric Acid Measurements in Serum
Uric acid was assessed in serum samples from a subset of individuals from the original four groups (Table 2). Differences in terms of age were noted; the median age range spanned 8 yr. There were fewer women in the control smoking group. Smoking habits were similar between the two smoking subgroups. Although hyperuricemia was evident in some subjects (both with and without AUDs), no significant differences in serum uric acid quantity were found to be present across these groups (P = 0.53, Wilcoxon’s/Kruskal-Wallis testing; Fig. 1B). Correlations among serum uric acid and age (P = 0.85), AUDIT score (P = 0.82), and pack-year smoking (P = 0.56) were not significant. However, as a group, men demonstrated higher serum uric acid values than did women (P = 0.003). Re-analyzing data across the four subject groups in men only did not suggest a relationship between serum uric acid with AUDs or smoking (P = 0.17).
Table 2.
Alcohol use disorder subjects and controls with serum uric acid measurements
| Control Nonsmokers | Control Smokers | AUD Nonsmokers | AUD Smokers | P | |
|---|---|---|---|---|---|
| n | 11 | 12 | 15 | 25 | |
| Age, yr | 36 (30–39) | 41 (37–47) | 44 (39–50) | 44 (38–48) | 0.003 |
| Sex (%men) | 8/11 (73%) | 5/12 (42%) | 13/15 (87%) | 23/25 (92%) | 0.005 |
| AUDIT score | 2 (1–4) | 2 (1–4) | 17 (13–32) | 28 (17–37) | <0.0001 |
| Pack-year smoking | n/a | 14 (9–25) | n/a | 15 (4–19) | 0.58 |
Values are medians (interquartile range). AUD, alcohol use disorder; AUDIT, AUD identification test.
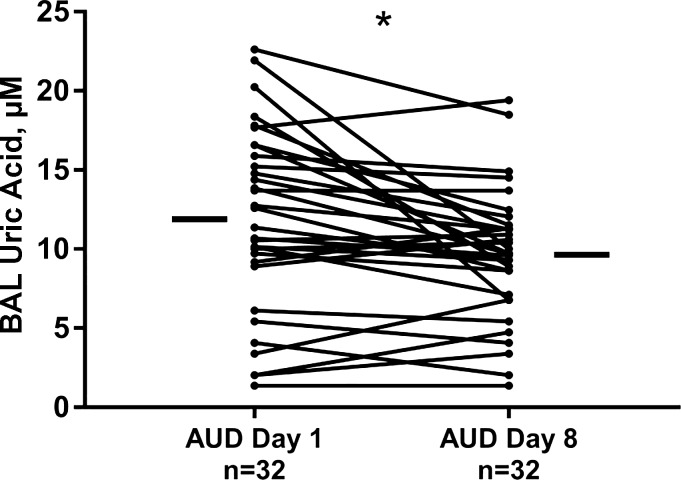
Paired Analyses of ELF Uric Acid Values
ELF obtained from BAL, collected in two bronchoscopy procedures, separated by 7 days of directly observed abstinence from alcohol was analyzed for uric acid content using samples from 32 subjects with AUDs. ELF uric acid values did decrease with abstinence (P = 0.003; Fig. 2). However, uric acid values, measured after 7 days abstinence (i.e., on day 8) among individuals with AUDs, remained substantially more elevated compared with ELF uric acid measured in controls [9.7 (IQR 6.9–11.5) μM in AUDs at day 8 vs. 2.0 (IQR 1.0–3.0) μM in nonsmoking controls and 3.4 (IQR 2.7–4.7) μM in smoking controls, P < 0.0001]. Serum uric acid values between these two time points did not appreciably change (P = 0.95 in paired analyses).
Fig. 2.
Epithelial lining fluid [ELF; derived from bronchoalveolar lavage (BAL) fluid] uric acid was measured in subjects with alcohol use disorders (AUDs; n = 32) within 48 h of the last alcohol-containing beverage and again after 7 days of observed abstinence on an inpatient unit on day 8. ELF uric acid values diminished with abstinence (*P < 0.003, Wilcoxon’s matched-pairs signed-rank test). Small, horizontal bars indicate median values of ELF uric acid at each time point.
XOR Activity in Freshly Collected BAL Cells and Serum
Prospectively collected fresh (nonfrozen) samples, consecutively obtained from AUD subjects and controls, were used to assess XOR activity. Cells were isolated from whole BAL samples and serum from whole blood before XOR activity analyses. BAL cell counts did not differ between AUD subjects and controls, and differential examination of BAL cells via microscopy revealed the predominant (>90%) cell type to be alveolar macrophages in both AUD subjects and controls. Moreover, peripheral white blood cell counts and differentials did not vary according to alcohol-use history.
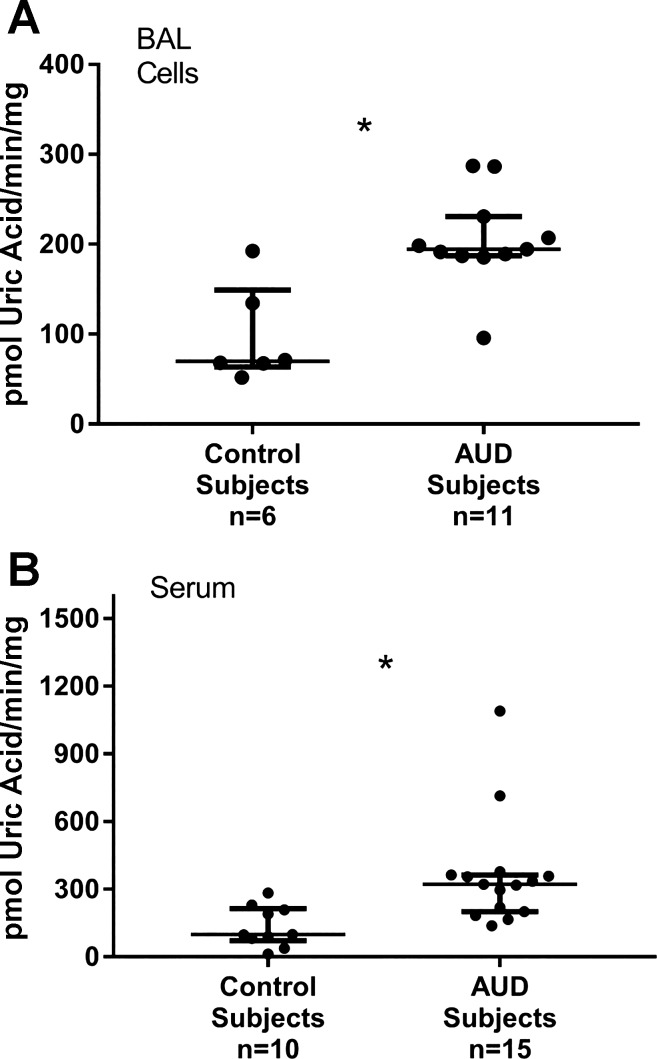
BAL cell XOR activity was measured in samples from control subjects (n = 6) and AUD subjects (n = 11) who did not significantly differ in terms of age, sex, or active smoking habits. XOR activity was detectable, and median values were noted to be approximately twofold higher in subjects with AUDs (P = 0.008; Fig. 3A). Serum XOR activity was measured in fresh samples from controls (n = 10) and AUD subjects (n = 15). Ages of AUD subjects were somewhat older than controls [median 37 (32–39) vs. 43 (39–46), P < 0.01], but the two groups were otherwise matched in terms of sex and smoking habits. Serum XOR activity was detectable in all subjects’ samples, but in contrast to what was observed with analyses of serum uric acid, serum XOR activity was roughly threefold more elevated in subjects with AUDs (P = 0.001; Fig. 3B). In paired analyses of XOR activity in serum and BAL cells, performed in samples from 13 individuals who had XOR activity measured in both sample types, serum XOR activity was somewhat greater than BAL cell XOR activity but was not statistically different between the two sample types (P = 0.15).
Fig. 3.
Xanthine oxidoreductase (XOR) activity in fresh cells from bronchoalveolar lavage (BAL), predominantly alveolar macrophages, and serum from control and alcohol use disorder (AUD) subjects, who were age, sex, and smoking history matched, was measured within 3 h of the procedure. XOR activity was determined by quantitating oxypurinol-inhibitable uric acid synthesis via spectrophotometry, adjusted for total protein in the sample. A: AUD subjects’ BAL cells displayed significantly higher XOR activity than did controls (*P = 0.008). B: AUD subjects displayed higher XOR activity in serum than did controls (*P = 0.001). Medians with IQRs are represented; Wilcoxon’s/Kruskal-Wallis testing was used for analysis.
Expression of XOR and Related Proteins in Isolated BAL Cells
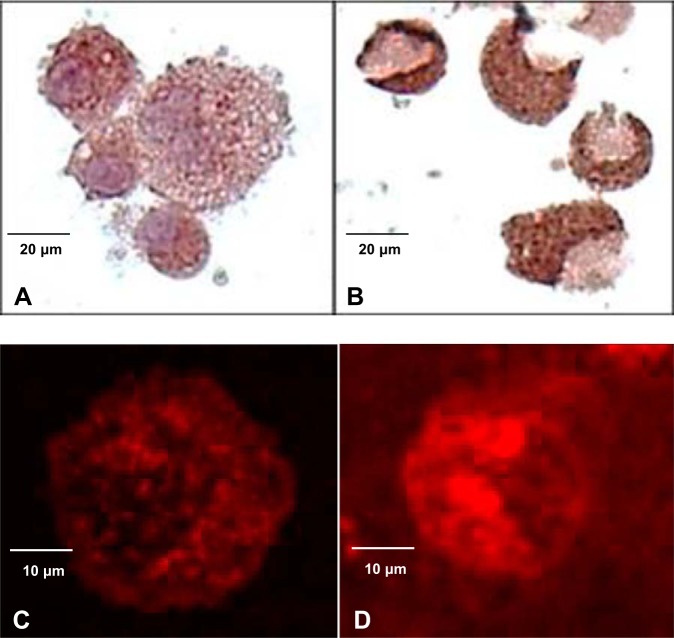
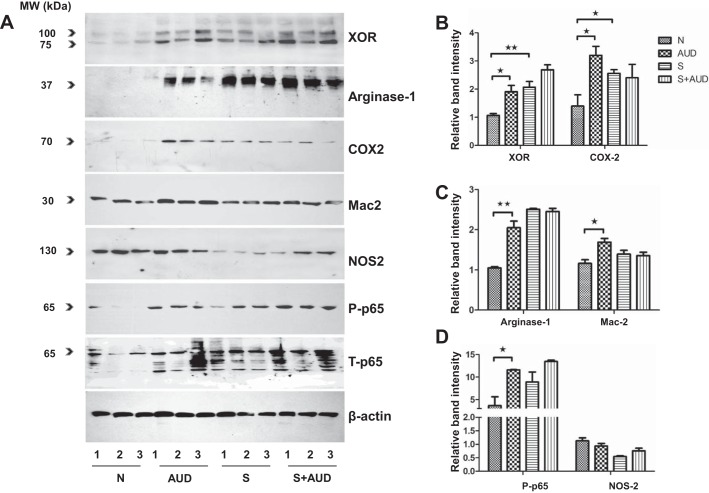
Immunohistochemical and immunofluorescent staining of cytospins prepared from BAL cells revealed prominent cytoplasmic staining for XOR in BAL cells, which was enhanced in subjects with AUDs (Fig. 4). Differential expression of proteins known to be regulated by XOR was subsequently examined in isolated BAL cells from the four groups of subjects, matched on the basis of age and smoking. Western immunoblot of total cell extracts from BAL revealed immunoreactive bands at 100 and 75 kDa when probing with anti-XOR antibodies, paralleling the higher XOR enzyme activity in these subject groups. The probing of membranes using the anti-COX-2 antibody revealed bands at 70 kDa that were increased in AUD subjects compared with controls; bands at 65 kDa reactive to the anti-phospho-NF-κB p65 antibody were also increased in the AUD setting, particularly among nonsmokers (Figs. 5, B and D). Additional Western immunoblot of total cell extracts revealed immunoreactive bands at 37 kDa reactive to the anti-Arg-1 antibody, which were increased in the AUD nonsmokers compared with control nonsmokers. Immunoreactive bands at 30 kDa using the anti-Mac-2 antibodies were also increased in AUD nonsmokers. Both Arg-1 and Mac-2 expression was comparable in smokers, regardless of AUD history (Fig. 5C).
Fig. 4.
Cytospins prepared from fresh bronchoalveolar lavage samples underwent immunohistochemical (IHC) and immunofluorescent (IF) staining to assess xanthine oxidoreductase (XOR) protein expression; representative photographs are shown. Cells from controls (A) and subjects with alcohol use disorders (AUDs; B) expressed cytoplasmic XOR protein by IHC; XOR staining was more intense in AUD subjects’ cells. Similarly, cells from controls (C) and subjects with AUDs (D) expressed cytoplasmic XOR protein by IF with more intense stain noted in the AUD subjects’ cells. IHC staining, 40× original magnification; IF, 80× magnification.
Fig. 5.
Western immunoblotting was used to quantify proteins in total cell extracts from bronchoalveolar lavage cells obtained from nonsmoking controls (“N,” n = 5), nonsmoking alcohol use disorder subjects (“AUD,” n = 5), smoking controls (“S,” n = 9), and smoking AUD subjects (“S + AUD,” n = 9). Three representative samples in each group are presented in the blots. β-Actin was also measured in the samples, and used to normalize values measured for proteins of interest. A: whereas the immunoblot against total NF-κB subunit p65 (T-p65) shows extensive modifications of the protein in AUD subjects, as well as smoking control and smoking AUD subjects, the antibody against the phosphorylated NF-κB p65 protein (P-p65) shows a specific single band at 65 kDa that is significantly increased in AUD subjects compared with their respective controls. NOS2, nitric oxide synthase 2. B–D: comparison of densitometric data for proteins of interest normalized to β-actin, xanthine oxidoreductase (XOR) protein, arginase-1, P-p65 protein, cyclooxygenase (COX)-2, and Mac-2 was significantly higher among nonsmoking subjects with AUDs (P < 0.05) compared with nonsmoking controls. In control smokers compared with control nonsmokers, COX-2 and XOR expression was also higher (*P < 0.05; **P < 0.01). NOS2 did not vary significantly across the 4 groups. Wilcoxon’s/Kruskal-Wallis testing was used for comparisons; means ± SE presented.
NLRP3 Inflammasome and Concomitant IL-1β Production in BAL Cells
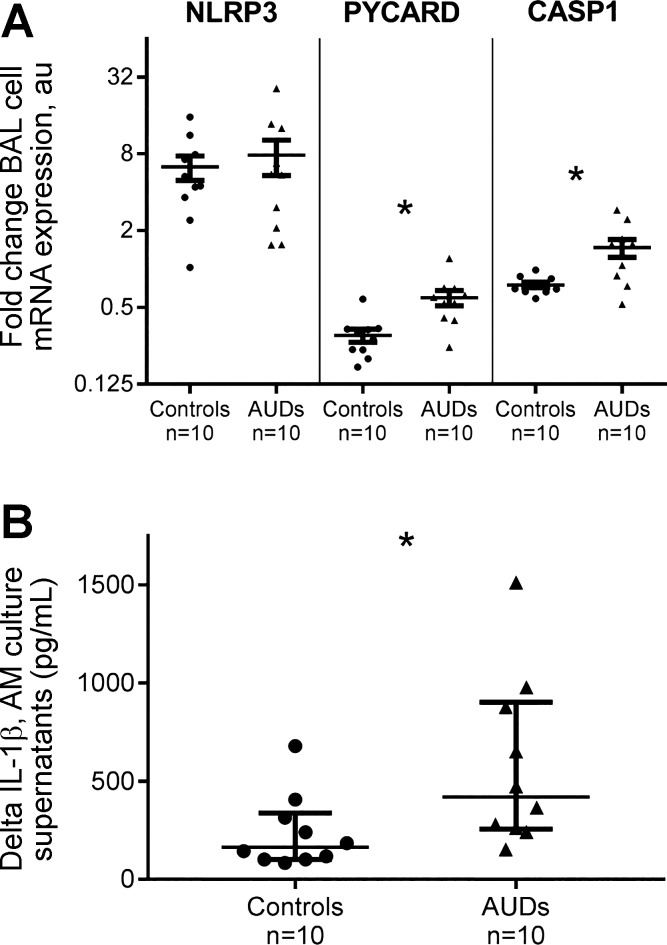
Alterations in gene expression for NLRP3, PYCARD, and CASP1 message expression by fresh BAL cells ex vivo in response to LPS stimulation were assessed. In response to LPS, BAL cells from AUD subjects and controls (n = 10 in each group, matched on age and sex, all nonsmokers) each exhibited an approximate fivefold increase in NLRP3 mRNA expression that was not significantly different [4.9-fold (IQR 3.3–8.7) relative expression in controls vs. 5.5-fold (IQR 2.0–12.9) expression in AUD subjects, P = 0.92; Fig. 6A]. In response to 18 h LPS stimulation, PYCARD mRNA expression was downregulated in both subject types; however, it remained approximately two times higher in BAL cells from subjects with AUDs at the 18-h time point [0.30-fold (IQR 0.22–0.34) change in controls vs. 0.57-fold (IQR 0.41–0.70) change in AUDs, P < 0.001]. In parallel, CASP1 mRNA expression in BAL cells from AUD subjects was relatively increased in response to LPS stimulation at the 18-h time point and was twofold greater than control BAL cells [0.71-fold (IQR 0.66–0.85) in controls vs. 1.44-fold (IQR 0.85–1.9) change in AUD subjects, P < 0.001].
Fig. 6.
Fresh cells from bronchoalveolar lavage [BAL; >90% alveolar macrophages (AM)] from control and AUD subjects (age and sex matched, all nonsmokers) were cultured, with and without the addition of LPS for 18 h. A: qRT-PCR was used to establish changes in BAL cell gene expression resulting from LPS stimulation (1 μg/ml) ex vivo; changes in expression of nucleotide-binding domain, leucine-rich-containing family, pyrin-domain containing-3 (NLRP3), apopotosis-associated speck-like protein with a caspase-1 (CASP1) recruitment domain (PYCARD or ASC), and CASP1 genes were subsequently assessed from collected cells. NLRP3 expression was increased in BAL cells from both controls and subjects with AUDs after 18 h LPS stimulation (P = not significant). PYCARD expression was diminished after 18 h LPS stimulation relative to no stimulation (media-only condition) in both subject types but remained significantly higher in AUD subjects compared with control subjects at this time point (*P < 0.009). CASP1 mRNA expression remained significantly higher in AUD subjects’ BAL cells compared with controls (*P < 0.001). Each subject had mRNA expression values measured in triplicate; fold changes in gene expression (means and SE) are presented. Analyses performed using Wilcoxon’s/Kruskal-Wallis testing. B: BAL cell-culture supernatants were collected at 18 h from both cells cultured in media only and cells cultured in the presence of LPS; IL-1β was assessed on a luminometric platform, and the difference in IL-1β production by BAL cells, with and without LPS stimulation, was calculated. BAL cells from AUD subjects secreted more IL-1β with LPS stimulation than did control subjects (*P = 0.02, Wilcoxon’s/Kruskal-Wallis testing). Samples were measured in duplicate.
BAL cell-culture supernatants, obtained from the above-described experiments that had been collected at 18 h from cells in the media-only condition and from cells cultured with LPS, were analyzed for IL-1β content. IL-1β quantity in culture supernatants in response to LPS stimulation was substantially more elevated in AUD subjects than controls [difference of 164 (IQR 102–337) pg/ml in controls vs. 419 (IQR 256–902) pg/ml in AUD subjects, P = 0.02; Fig. 6B].
DISCUSSION
Decades previously, Charles Lieber (34) observed systemic elevations of uric acid in humans with AUDs, believed to be attributable to the increased generation of purine nucleotides from the metabolism of consumed alcohol. Our work extends these earlier observations, providing evidence that uric acid is disproportionately elevated in the ELF of the lung in subjects with AUDs compared with ELF from healthy controls matched for smoking. Furthermore, we determined that abstinence from alcohol for 7 days was not sufficient to normalize ELF uric acid in controls. XOR activity, measured in freshly collected BAL cells (>90% AMs), was substantially higher in AUD subjects compared with controls. Complementary to these observations, XOR activity in serum was also increased among subjects with AUDs, although serum uric acid was not higher among subjects with AUDs. In cells obtained from BAL from AUD subjects and controls, XOR protein was detectible and quantitatively more abundant in the AUD setting, concurrent with enhanced expression of inflammatory proteins that include COX-2. In LPS-stimulated BAL cells, PYCARD and CASP1 mRNA expression remained persistently elevated in AUD subjects’ samples in conjunction with higher IL-1β production. Our data suggest a relationship between AUDs and augmented XOR activity in lung and blood that drives uric acid synthesis and contributes to oxidative stress. Given the potential for both uric acid and ROS to enhance inflammation through a variety of mechanisms, these alcohol-induced alterations could predispose individuals with AUDs to develop an abnormally robust inflammatory response in the setting of lung infection or ARDS and contribute to the increased morbidity seen clinically.
XOR catalyzes the terminal two steps of purine degradation and is the sole source of both ROS and uric acid that have been implicated in inflammatory lung diseases. XOR activity and ROS generation are regulated by cytokines, oxygen tension, and cellular redox state (20). COX-2, as a proinflammatory mediator, is involved in cancer progression, but its metabolites are also involved in regulating the redox status of inflammatory cells and modulation of inflammation that may involve the Kelch ECH-associating protein 1/NF erythroid 2-related factor (Nrf)2 pathway (26). Modulation of antioxidant and Nrf2 activity has been implicated in the pathogenesis of alcohol-induced lung inflammation (58); however, the exact mechanisms are not completely understood. Our group has previously demonstrated an important role for XOR in the modulation of COX-2 from mammary epithelial and breast carcinoma cells (16). Other reports noted a key role of XOR in modulating COX-2 expression in murine fibroblasts (43). The data we describe from BAL cells suggest a potential and novel role for XOR in modulation of the Kelch ECH-associating protein 1/Nrf2 pathway through effects on COX-2 and NF-κB expression (phosphorylated p65 subunit) that we found to be increased in parallel with XOR among subjects with AUDs who did not smoke. Additionally, changes in XOR activity and expression in AUD nonsmokers correlated with markers of macrophage inflammatory polarization, Arg-1 and Mac-2, in isolated BAL cells. Since these results were from a small number of individuals, future experiments will be necessary to validate these associations.
Although uric acid has been postulated to function in a pulmonary antioxidant capacity along with proteins, including glutathione (22, 40, 61), uric acid has also been found to be consistently elevated in several lung-injury models in association with enhanced inflammation and neutrophilia. XOR inhibition appears to abrogate these effects (17, 21, 28, 31, 63). In one investigation, uric acid directly activated the NLRP3 inflammasome, resulting in increased IL-1β and IL-18 expression in lung macrophages, suggesting that uric acid itself drives inflammation (17). A separate investigation indicated that XOR-derived ROS can also incite IL-1β release in macrophages that is diminished by a XOR blockade (27). Acute alcohol exposure has been shown to inhibit NLRP3 inflammasome activation in vitro (23) in cells that included human peripheral blood mononuclear cells. However, in studies conducted to investigate inflammasome activation, wild-type mice, fed the Lieber-DeCarli diet for 4 wk to mimic chronic alcohol exposure, exhibited increased uric acid levels in serum and liver, leading to IL-1β production and liver inflammation that appeared to be mediated by NLRP3 (25, 44). In separate investigations, chronic ethanol exposure also triggered Toll-like receptor 4-mediated NLRP3 inflammasome activation in glial cells that may alter the blood brain barrier’s permeability, promoting neuroinflammation (3). These latter investigations, conducted in animal models of chronic alcohol exposure, mirror our current human subjects’ investigations, where AUD subjects had a long-standing history of heavy alcohol consumption. Purine degradation, necessitated by alcohol metabolism and facilitated by XOR, generates hydrogen peroxide and superoxide, both of which promote oxidative stress. Pulmonary oxidative stress has been reported consistently in the setting of AUDs, where it may deleteriously affect the immune response supported by AMs; systemic oxidative stress has also been observed in this setting (65). Previous investigations by our group have indicated that AUDs are associated with an augmented inflammatory response by AMs to stimulus with LPS (18, 42). Notably, LPS-stimulated AMs from AUD subjects secreted increased quantities of IL-1β, as well as IL-6 and TNF-α. Importantly, concurrent addition of N-acetylcysteine, a precursor to the antioxidant glutathione, attenuated the proinflammatory response of AMs, suggesting that heightened activity by AMs to pathogen-associated molecular pattern stimulation may be governed by oxidative stress (18). In the current investigation, BAL cells from subjects with AUDs and controls each displayed increased NLRP3 message expression after LPS stimulation for 18 h; however, both PYCARD and CASP1 expression at this time point remained relatively elevated only in AUD samples. Moreover, confirming our previous work (18), IL-1β secretion by BAL cells was augmented among AUD subjects compared with controls, in parallel with heightened PYCARD and CASP1 expression. Certainly, differences in NLRP3 inflammasome mRNA expression at times before 18 h would more fully establish the time course of NLRP3 alterations in this setting. Additionally, the exploration of NLRP3 inflammasome expression in human cells, directly in response to uric acid, would be worthwhile.
Antioxidant replacement has been examined as one component of ARDS therapy with disappointing results (50). Exogenous administration of N-acetylcysteine in the setting of ARDS has also been examined in a more limited fashion (8, 57). Notably, these prior investigations have not specifically targeted patients with alcohol-associated ARDS, where such therapy could be more efficacious. In lieu of antioxidant replacement, therapeutic alternatives that diminish XOR activity would be novel in their capability to decrease production of ROS, as well as the damage-associated molecular pattern uric acid. Such specific clinically available XOR inhibitors include allopurinol and febuxostat. These agents are in widespread use for hyperuricemia, where they have an excellent safety profile (29). Our ability to measure XOR activity in freshly collected cells and serum from enrolled subjects also raises the possibility of developing XOR activity measures at the point of care to personalize XOR-targeted therapies. Notably, the time required to prepare samples to analyze XOR by spectrophotometry was relatively short (<1 h), and activity measurements (including replicates) were completed in <3 h total. Moreover, the similarity in magnitude of XOR activity between serum and BAL cells that we observed suggests the possibility that serum analyses may be sufficient to characterize XOR activity in the lung (or other organs). Emerging research in ARDS has highlighted the importance of acknowledging diverse patient phenotypes in considering therapeutic options (12, 14); it is expected that XOR inhibition may not be equally efficacious for all ARDS patients. Our ability to measure XOR activity nearly in real time will facilitate further examination of the relevance of this enzyme in ARDS development and outcomes, with an ultimate goal of clarifying the therapeutic potential for XOR inhibition in this setting. It is important to establish not only the impact of AUDs and smoking on XOR activity but also the impact of underlying conditions (both acute and chronic) or other systemic illness (e.g., sepsis) (5) to enable targeting of XOR, where it is likely to be of the most benefit. Of note, measures of XOR activity are truly specific for the enzyme’s activity compared with measures of uric acid, a by-product of XOR enzyme activity. Moreover, the large quantitative differences between uric acid in serum and ELF that we observed suggest that measures of serum uric acid would be less useful as a surrogate to determine XOR activity in other organs.
Our work is not without limitations. We were able to detect differences in ELF uric acid in stored biorepository specimens from subjects with AUDs and controls who were, by design, without demonstrable evidence of co-morbid conditions, suggesting that AUDs enhance XOR activity in the lung. However, age differences, sex, occult organ dysfunction, and race may have influenced these observations. Certainly, these factors could have also contributed to serum uric acid measured in AUD subjects and controls. XOR is known to be present in a variety of cell types; therefore, it remains possible that elevated ELF uric acid values observed in the AUD setting are related to differences in XOR activity within other lung cells (e.g., bronchial airway epithelial cells) or other organs, such as the liver (5, 25, 44). However, we are confident in the alcohol-use histories provided by our subjects, given their recruitment from a detoxification center where they are well known to staff. Additionally, alterations in uric acid over time were measured in subjects who were inpatient status and had no access to alcohol, strengthening the validity of our observations. We were unable to measure specific XOR enzyme activity in banked specimens, due to the requirements of the assay. However, to verify that XOR enzyme activity was specifically increased in the setting of AUDs, additional subjects were enrolled to obtain fresh BAL cells and serum; this subset of subjects was smaller, given the period of time required to obtain prospectively sufficient sample numbers in AUD and control subjects to analyze and compare XOR activity. XOR activity differences, measured in freshly collected specimens, could provide a strategy to target certain patients for enzyme-modulating activity but will require additional verification in a larger group of subjects. It is presently unclear if serum XOR activity mirrors XOR activity within the lung or other organs. Additional examination of XOR activity across organ systems within the same subject may help determine if serum analyses of XOR activity are sufficient to identify individuals with increased XOR activity in the lung who would be logical targets for XOR inhibitory therapy.
In conclusion, in a cohort of otherwise healthy individuals with AUDs, XOR activity within lung-immune cells, primarily AMs, and XOR activity in serum were significantly elevated compared with matched controls. Uric acid was also substantially elevated in ELF from subjects with AUDs. We believe that the activity of XOR contributes to oxidative stress in the AUD setting and is a targetable driver of the heightened inflammatory mediator production previously observed in AMs from these individuals. The understanding of the role of XOR as a contributor to lung dysfunction reported in AUDs may provide new therapeutic options to normalize lung immunity, prevent ARDS development, and improve outcomes in AUD-associated ARDS.
GRANTS
This study was funded by National Institutes of Health Grants R24AA019661 and UL1TR001082 and U.S. Department of Defense Grant W81XWH-14-1-0451.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.F. and E.L.B. conceived and designed research; M.A.F., J.G., A.M., and V.K. performed experiments; M.A.F. and E.L.B. analyzed data; M.A.F. and E.L.B. interpreted results of experiments; M.A.F. and E.L.B. prepared figures; M.A.F., J.G., A.M., V.K., and E.L.B. edited and revised manuscript; M.A.F. and E.L.B. approved final version of manuscript; E.L.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank the clients and staff at Denver CARES who assisted and participated in these investigations.
REFERENCES
- 1.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE III, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. doi: 10.1152/ajplung.00453.2005. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso-Loeches S, Ureña-Peralta J, Morillo-Bargues MJ, Gómez-Pinedo U, Guerri C. Ethanol-induced TLR4/NLRP3 neuroinflammatory response in microglial cells promotes leukocyte infiltration across the BBB. Neurochem Res 41: 193–209, 2016. doi: 10.1007/s11064-015-1760-5. [DOI] [PubMed] [Google Scholar]
- 4.Babor TF, Higgins-Biddle JC, Saunders JB, Montiero MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care (2nd ed.). Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence, 2006, p. 11–22. [Google Scholar]
- 5.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochim Biophys Acta 1842: 1502–1517, 2014. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, Lademacher C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 12: R63, 2010. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353: 2450–2461, 2005. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE; The Antioxidant in ARDS Study Group . A trial of antioxidants N-acetylcysteine and procysteine in ARDS. Chest 112: 164–172, 1997. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327–336, 2004. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 9a.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Burnham EL, Brown LA, Halls L, Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res 27: 1167–1172, 2003. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- 11.Burnham EL, McCord JM, Bose S, Brown LA, House R, Moss M, Gaydos J. Protandim does not influence alveolar epithelial permeability or intrapulmonary oxidative stress in human subjects with alcohol use disorders. Am J Physiol Lung Cell Mol Physiol 302: L688–L699, 2012. doi: 10.1152/ajplung.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA; NHLBI ARDS Network . Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2: 611–620, 2014. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark BJ, Williams A, Feemster LM, Bradley KA, Macht M, Moss M, Burnham EL; NHLBI ARDS Network Investigators . Alcohol screening scores and 90-day outcomes in patients with acute lung injury. Crit Care Med 41: 1518–1525, 2013. doi: 10.1097/CCM.0b013e318287f1bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS. ARDS subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 195: 331–338, 2017. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fini MA, Monks J, Farabaugh SM, Wright RM. Contribution of xanthine oxidoreductase to mammary epithelial and breast cancer cell differentiation in part modulates inhibitor of differentiation-1. Mol Cancer Res 9: 1242–1254, 2011. doi: 10.1158/1541-7786.MCR-11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fini MA, Orchard-Webb D, Kosmider B, Amon JD, Kelland R, Shibao G, Wright RM. Migratory activity of human breast cancer cells is modulated by differential expression of xanthine oxidoreductase. J Cell Biochem 105: 1008–1026, 2008. doi: 10.1002/jcb.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med 179: 903–913, 2009. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 18.Gaydos J, McNally A, Guo R, Vandivier RW, Simonian PL, Burnham EL. Alcohol abuse and smoking alter inflammatory mediator production by pulmonary and systemic immune cells. Am J Physiol Lung Cell Mol Physiol 310: L507–L518, 2016. doi: 10.1152/ajplung.00242.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbings S, Elkins ND, Fitzgerald H, Tiao J, Weyman ME, Shibao G, Fini MA, Wright RM. Xanthine oxidoreductase promotes the inflammatory state of mononuclear phagocytes through effects on chemokine expression, peroxisome proliferator-activated receptor-gamma sumoylation, and HIF-1alpha. J Biol Chem 286: 961–975, 2011. doi: 10.1074/jbc.M110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33: 774–797, 2002. doi: 10.1016/S0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 21.Hassoun PM, Yu FS, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1, and hypoxia. Role in acute lung injury. Am J Respir Crit Care Med 158: 299–305, 1998. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- 22.Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax 69: 1021–1026, 2014. doi: 10.1136/thoraxjnl-2014-205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyt LR, Ather JL, Randall MJ, DePuccio DP, Landry CC, Wewers MD, Gavrilin MA, Poynter ME. Ethanol and other short-chain alcohols inhibit NLRP3 inflammasome activation through protein tyrosine phosphatase stimulation. J Immunol 197: 1322–1334, 2016. doi: 10.4049/jimmunol.1600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol 97: 149–206, 1979. [PMC free article] [PubMed] [Google Scholar]
- 25.Iracheta-Vellve A, Petrasek J, Satishchandran A, Gyongyosi B, Saha B, Kodys K, Fitzgerald KA, Kurt-Jones EA, Szabo G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 63: 1147–1155, 2015. doi: 10.1016/j.jhep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol Cell Biol 24: 36–45, 2004. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, Simon G, Busso N, So A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat Commun 6: 6555, 2015. doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka H, Yang K, Rock KL. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury-induced inflammation in the liver and lung. Eur J Pharmacol 746: 174–179, 2015. doi: 10.1016/j.ejphar.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley EE. Dispelling dogma and misconceptions regarding the most pharmacologically targetable source of reactive species in inflammatory disease, xanthine oxidoreductase. Arch Toxicol 89: 1193–1207, 2015. doi: 10.1007/s00204-015-1523-8. [DOI] [PubMed] [Google Scholar]
- 30.Kelley EE, Hock T, Khoo NK, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR Jr, Tarpey MM. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Radic Biol Med 40: 952–959, 2006. doi: 10.1016/j.freeradbiomed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Kuipers MT, Aslami H, Janczy JR, van der Sluijs KF, Vlaar AP, Wolthuis EK, Choi G, Roelofs JJ, Flavell RA, Sutterwala FS, Bresser P, Leemans JC, van der Poll T, Schultz MJ, Wieland CW. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology 116: 1104–1115, 2012. doi: 10.1097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers MT, Aslami H, Vlaar AP, Juffermans NP, Tuip-de Boer AM, Hegeman MA, Jongsma G, Roelofs JJ, van der Poll T, Schultz MJ, Wieland CW. Pre-treatment with allopurinol or uricase attenuates barrier dysfunction but not inflammation during murine ventilator-induced lung injury. PLoS One 7: e50559, 2012. doi: 10.1371/journal.pone.0050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122, Suppl: 314S–320S, 2002. doi: 10.1378/chest.122.6_suppl.314S. [DOI] [PubMed] [Google Scholar]
- 34.Lieber CS. Hyperuricemia induced by alcohol. Arthritis Rheum 8: 786–798, 1965. doi: 10.1002/art.1780080442. [DOI] [PubMed] [Google Scholar]
- 35.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561–568, 2011. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 275: 50–54, 1996. doi: 10.1001/jama.1996.03530250054027. [DOI] [PubMed] [Google Scholar]
- 38.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 161: 414–419, 2000. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 39.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 31: 869–877, 2003. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 40.Nicks ME, O’Brien MM, Bowler RP. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD 8: 264–269, 2011. doi: 10.3109/15412555.2011.579202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomura J, Busso N, Ives A, Matsui C, Tsujimoto S, Shirakura T, Tamura M, Kobayashi T, So A, Yamanaka Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci Rep 4: 4554, 2014. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Halloran EB, Curtis BJ, Afshar M, Chen MM, Kovacs EJ, Burnham EL. Alveolar macrophage inflammatory mediator expression is elevated in the setting of alcohol use disorders. Alcohol 50: 43–50, 2016. doi: 10.1016/j.alcohol.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res 95: 1118–1124, 2004. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 44.Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, Kurt-Jones EA, Szabo G. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol 98: 249–256, 2015. doi: 10.1189/jlb.3AB1214-590R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, Gattas DJ, Hallett D, Tomlinson G, Stewart TE, Ferguson ND. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 179: 220–227, 2009. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 46.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 194: 147–155, 2016. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med 155: 479–484, 1997. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- 48.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res 26: 272–279, 2002. doi: 10.1111/j.1530-0277.2002.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 50.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P; NIH NHLBI Acute Respiratory Distress Syndrome Network of Investigators . Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 306: 1574–1581, 2011. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 307: 795–803, 2012. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, Poli G, Viña J. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med 49: 171–177, 2010. doi: 10.1016/j.freeradbiomed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 54.Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest 74: 48–56, 1996. [PubMed] [Google Scholar]
- 55.Schmidt R, Luboeinski T, Markart P, Ruppert C, Daum C, Grimminger F, Seeger W, Günther A. Alveolar antioxidant status in patients with acute respiratory distress syndrome. Eur Respir J 24: 994–999, 2004. doi: 10.1183/09031936.04.00120703. [DOI] [PubMed] [Google Scholar]
- 56.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 57.Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, Reza Khajavi M, Reza Rouini M, Moradi M, Mohammadirad A, Abdollahi M. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol 26: 697–703, 2007. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- 58.Sueblinvong V, Tseng V, Smith T, Saghafi R, Mills ST, Neujahr DC, Guidot DM. TGFβ1 mediates alcohol-induced Nrf2 suppression in lung fibroblasts. Alcohol Clin Exp Res 38: 2731–2742, 2014. doi: 10.1111/acer.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10: 739–753, 2008. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 60.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS, deBoisblanc BP, Hough CL, Hite RD, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 370: 2191–2200, 2014. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol 276: L289–L296, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 63.Wright RM, Ginger LA, Kosila N, Elkins ND, Essary B, McManaman JL, Repine JE. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am J Respir Cell Mol Biol 30: 479–490, 2004. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Moriwaki Y, Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta 356: 35–57, 2005. doi: 10.1016/j.cccn.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 65.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 176: 270–276, 2007. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120–1127, 2008. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]