Figure 1.
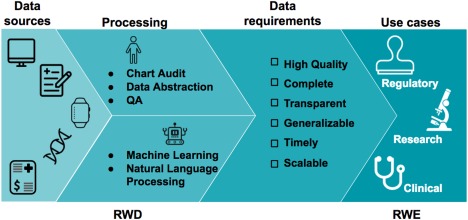
The journey from data to evidence. Real‐world data (RWD) are data that are routinely collected in the form of electronic health records (EHRs), patient disease registries, wearables, genomic datasets, medical claims registries, and others. These data can be aggregated, linked, and processed to produce key conclusions in the form of real‐world evidence (RWE). The proposed checklist can be used to assess if the quality of the RWD is regulatory‐grade.
