Figure 2.
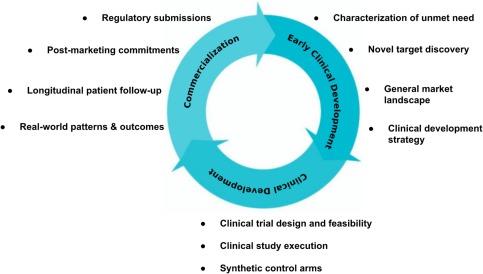
Use cases for RWE in the drug development cycle. RWE can be valuable across the lifecycle of drug development. In a drug's predevelopment stages, RWE helps inform clinical development strategies and study planning. During clinical development stages, RWE may inform trial design and feasibility (e.g., patient enrollment, inclusion and exclusion criteria), and serve as a synthetic control arm. Following drug approval, RWE may help fulfill postmarketing commitments and potentially support label expansions. Finally, in clinical practice, RWE may assist personalization of treatment by offering clinicians and patients a deeper understanding of the nuances of drug performance in the real world.
