Figure 1.
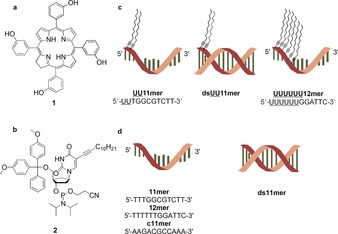
Representation of a) meta‐tetra‐hydroxyphenyl‐chlorin (mTHPC (1)); b) 5‐(dodec‐1‐ynyl)uracil deoxyribophosphoramidite (2) used in solid‐phase synthesis of lipid‐DNAs, this nucleotide building block is abbreviated as U in the corresponding sequences; c) lipid‐DNAs (UU11 mer, double‐stranded UU11 mer (dsUU11 mer) and UUUUUU12 mer) used for the solubilization of 1; d) pristine control DNAs (11 mer, complementary 11 mer (c11 mer), double‐stranded 11 mer (ds11 mer) and 12 mer).
