Table 1.
Optimization studies.[a]
| Entry | Additive | Yield[b] [%] | d.r.[c] | ee [d] [%] |
|---|---|---|---|---|
| 1[e] | – | 53 | 8:1 | 95 |
| 2[e,f] | – | 0 | – | – |
| 3 | – | 65 | 8:1 | 97 |
| 4 | BP (1 equiv) | 88 | 8:1 | 97 |
| 5 | BP (0.2 equiv) | 75 | 8:1 | 97 |
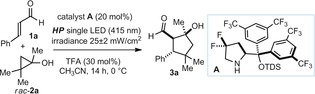
[a] Reactions performed at 0 °C on a 0.1 mmol scale using 2 equiv of 1 a under illumination with a single high‐power (HP) LED (λ max=415 nm) with an irradiance of 25 mW cm−2. [b] Yield of 3 a isolated as a mixture of diastereomers. [c] Diastereomeric ratio inferred by 1H NMR analysis of the crude mixture. [d] Enantiomeric excess of 3 a determined by UPC2 analysis on a chiral stationary phase. [e] Performed at ambient temperature. [f] In the dark. BP=1,1′‐biphenyl, TDS=thexyldimethylsilyl, TFA=trifluoroacetic acid.
