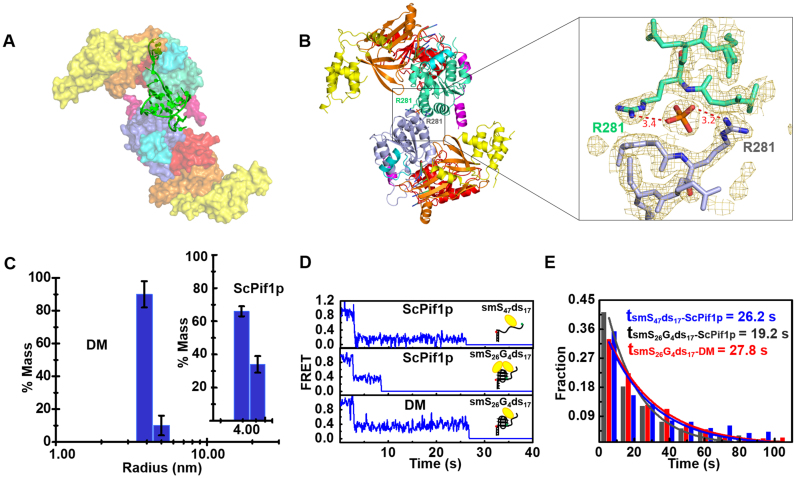
Figure 4.
(A) The SAXS model of ScPif1p dimer stabilized by 8T3G4. (B) Identified crystal structure of dimeric ScPif1p and a close-up view of the interface between the two monomers. (C) Radius distributions of ScPif1p (inset) and the DM mutant obtained from analyses of experimental data in DLS assays. The experiments were performed as described in ‘Materials and Methods’ with 4.5 μM proteins. (D) Individual smFRET traces recorded in experiments performed with a partial duplex DNA (smS47ds17, Supplementary Table S1) or a partial duplex DNA bearing a G4 motif at the ss/dsDNA junction (smS26G4ds17, Supplementary Table S1), in the presence of ScPif1p (upper and middle panels) or the DM mutant (lower panel) in which the dimeric interaction is disrupted. The detailed experimental conditions are described in ‘Materials and Methods’. (E) Histograms of waiting time constructed from about 300 individual records of ScPif1p with smS47ds17 or smS26G4ds17, and DM with smS26G4ds17. They follow an exponential decay with time constants of 26.2, 19.2 and 27.8 s, respectively.