Figure 1.
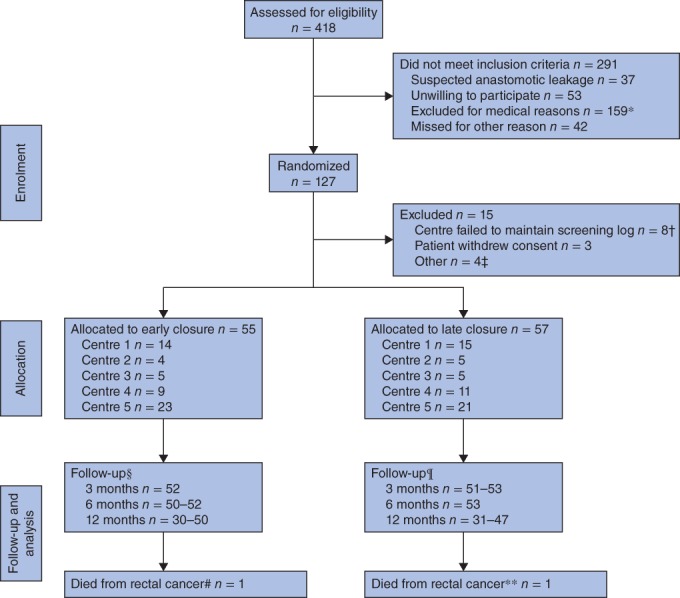
Participant flow diagram, as in the EASY trial17. *Paralytic ileus (24), Hartmann procedure with intersphincteric dissection (16), delayed postoperative recovery (15), perioperative complications (7), other infection (5), reoperation (7), high stoma output (5), pulmonary embolism (1), ulcerative colitis (1), extensive cancer disease (3), cardiovascular disease (2), language difficulty (5), diabetes (28), permanent or no stoma (29), steroid treatment (3), other (8). †Centre 6 (2), centre 7 (3), centre 8 (3). ‡Allocated to early closure, but not possible to perform surgery within 8–13 days (1); early closure outside the study (2); randomized, but no further information available (1). §3 months: Short Form 36 (SF‐36®) (52), European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 (52), EORTC QLQ‐CR29 (52); 6 months: 3 months: SF‐36® (52), EORTC QLQ‐C30 (50), EORTC QLQ‐CR29 (50); 12 months: 3 months: SF‐36® (50), EORTC QLQ‐C30 (30), EORTC QLQ‐CR29 (50). ¶3 months: SF‐36® (53), EORTC QLQ‐C30 (51), EORTC QLQ‐CR29 (52); 6 months: 3 months: SF‐36® (53), EORTC QLQ‐C30 (53), EORTC QLQ‐CR29 (53); 12 months: SF‐36® (47), EORTC QLQ‐C30 (31), EORTC QLQ‐CR29 (47). #Missing from follow‐up at 12 months. **Patient did not have closure; missing from follow‐up at 12 months
