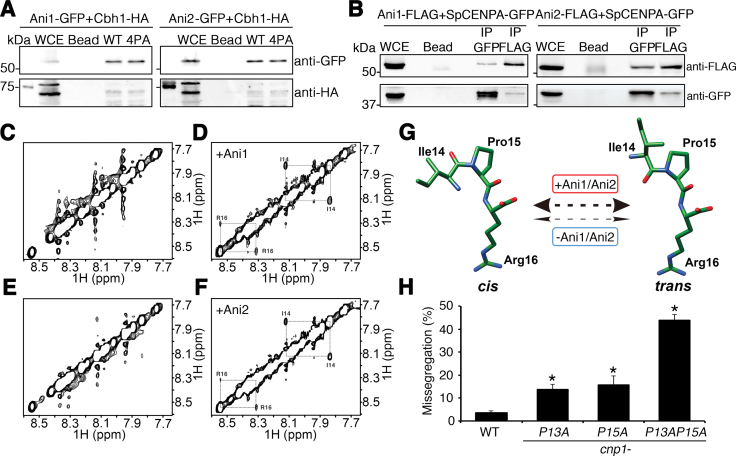
Figure 3.
Cis–trans conformational change of SpCENP-A NTD by Ani1 and Ani2 peptidyl prolyl isomerases. (A) Binding of Ani1-GFP (left) and Ani2-GFP (right) to wild type (WT) and 4PA SpCENP-A amino-terminal domain (NTD) peptides. Ani1 and Ani2 were detected by immunoblotting with anti-GFP. WCE, whole cell extract; Bead, beads only control; Cbh1-HA, positive IP and loading control. (B) Co-immunoprecipitation of Ani1-FLAG (left) or Ani2-FLAG (right) to wild type SpCENP-A-GFP in vivo. IP GFP, immunoprecipitation performed with anti-GFP; IP FLAG, immunoprecipitation performed with anti-FLAG. Representative blot of three experimental replicates. (C–F) Two-dimensional 1H rotating frame Overhauser effect spectroscopy (ROESY) nuclear magnetic resonance (NMR) profiles of SpCENP-A NTD peptides in the absence (C, E) and presence (D, F) of recombinant Ani1 (C, D) and Ani2 (E, F). Diagonal cis–trans cross-peaks at Isoleucine (I)-14 and Arginine (R)-16 were obtained for both Ani1 and Ani2. (G) Structural model depicting isomerization catalyzed by Ani1 and Ani2 (thick double-headed arrow) centering on Proline (Pro)15 in the SpCENP-A NTD. The rate of isomerization is negligible in the absence of the enzyme (thin double-headed arrows). (H) Chromosome missegregation frequency of WT and cnp1-P13A, cnp1-P15A and cnp1-P13AP15A mutants. Two tailed t-test, *P < 0.05, N = 300. Error: S.D. Bar plot indicates the mean of three biological replicates.