Key Points
Question
Does baseline nutritional status explain the lack of an effect of multivitamin use on risk of cardiovascular disease in the Physicians’ Health Study II?
Findings
In this randomized clinical trial of 13 316 male physicians observed for a mean (SD) follow-up of 11.4 (2.3) years, there was no consistent evidence of effect modification by various foods, nutrients, dietary patterns, or baseline supplement use on the effect of multivitamin use on cardiovascular disease end points.
Meaning
Long-term multivitamin use does not prevent major cardiovascular disease events in men, regardless of baseline nutritional status.
Abstract
Importance
Long-term multivitamin use had no effect on risk of cardiovascular disease (CVD) in the Physicians’ Health Study II. Baseline nutritional status may have modified the lack of effect.
Objective
To investigate effect modification by various baseline dietary factors on CVD risk in the Physicians’ Health Study II.
Design, Setting, and Participants
The Physicians’ Health Study II was a randomized, double-blind, placebo-controlled trial testing multivitamin use (multivitamin [Centrum Silver] or placebo daily) among US male physicians. The Physicians’ Health Study II included 14 641 male physicians 50 years or older, 13 316 of whom (91.0%) completed a baseline 116-item semiquantitative food frequency questionnaire and were included in the analyses. This study examined effect modification by baseline intake of key foods, individual nutrients, dietary patterns (Alternate Healthy Eating Index and Alternate Mediterranean Diet Score), and dietary supplement use. The study began in 1997, with continued treatment and follow-up through June 1, 2011.
Interventions
Multivitamin or placebo daily.
Main Outcomes and Measures
Major cardiovascular events, including nonfatal myocardial infarction, nonfatal stroke, and CVD mortality. Secondary outcomes included myocardial infarction, total stroke, CVD mortality, and total mortality individually.
Results
In total, 13 316 male physicians (mean [SD] age at randomization, 64.0 [9.0] years in those receiving the active multivitamin and 64.0 [9.1] years in those receiving the placebo) were observed for a mean (SD) follow-up of 11.4 (2.3) years. There was no consistent evidence of effect modification by various foods, nutrients, dietary patterns, or baseline supplement use on the effect of multivitamin use on CVD end points. Statistically significant interaction effects were observed between multivitamin use and vitamin B6 intake on myocardial infarction, between multivitamin use and vitamin D intake on CVD mortality, and between multivitamin use and vitamin B12 intake on CVD mortality and total mortality. However, there were inconsistent patterns in hazard ratios across tertiles of each dietary factor that are likely explained by multiple testing.
Conclusions and Relevance
The results suggest that baseline nutritional status does not influence the effect of randomized long-term multivitamin use on major CVD events. Future studies are needed to investigate the role of baseline nutritional biomarkers on the effect of multivitamin use on CVD and other outcomes.
Trial Registration
clinicaltrials.gov Identifier: NCT00270647
This secondary analysis of the Physicians’ Health Study II randomized clinical trial investigates effect modification by various baseline dietary factors on cardiovascular disease risk.
Introduction
Multivitamin dietary supplements usually contain most of the essential vitamins and minerals in amounts that tend to mirror those optimally obtained through diet, and their use aims to prevent nutritional deficiencies or insufficiencies. More than 50% of older US adults take a multivitamin supplement, often with the intent to improve or maintain health. However, the evidence of health benefits from multivitamin use remains scarce, with the US Preventive Services Task Force concluding that there is insufficient evidence to recommend for or against use of multivitamins to prevent cardiovascular disease (CVD).
Observational prospective and case-control cohort studies have reported mixed results on multivitamin use and risk of CVD, likely reflecting diversity in study populations, outcomes, types of multivitamins used, and residual confounding because multivitamin users tend to have particular lifestyle and dietary patterns. Randomized clinical trials are the optimal study design to eliminate confounding and determine causality. The Physicians’ Health Study II (PHS II), the only completed randomized clinical trial to date testing the effect of a common broad-spectrum, low-dose multivitamin on CVD, included 14 641 male physicians 50 years or older and previously reported no overall effect of multivitamin use on major CVD events during a median of 11.2 years of follow-up, but it significantly reduced the incidence of total cancer and cataract. In addition, baseline age appeared to modify the effect of multivitamin use on CVD where men 70 years or older had a nonsignificant lower risk of major CVD events. However, little is known about the role of multivitamins in CVD prevention among individuals with increased nutrient requirements.
Therefore, we investigated whether baseline nutritional status may explain the lack of an overall effect of multivitamin use on CVD in the PHS II. We sought to understand whether baseline intake of key foods, nutrients, dietary patterns, and dietary supplements modified the long-term effect of multivitamin use on major CVD and mortality in the PHS II.
Methods
Study Population
The PHS II has been described previously. In brief, the PHS II was a randomized, double-blind, placebo-controlled 2 × 2 × 2 × 2 factorial trial evaluating the effects of the following supplements in the prevention of cancer, CVD, eye disease, and cognitive decline: a multivitamin (multivitamin [Centrum Silver] or placebo daily) (eTable in Supplement 1), vitamin E (400 IU of synthetic alpha-tocopherol or placebo on alternate days), vitamin C (500 mg of synthetic ascorbic acid or placebo daily), and beta-carotene (50 mg of beta-carotene or placebo on alternate days).
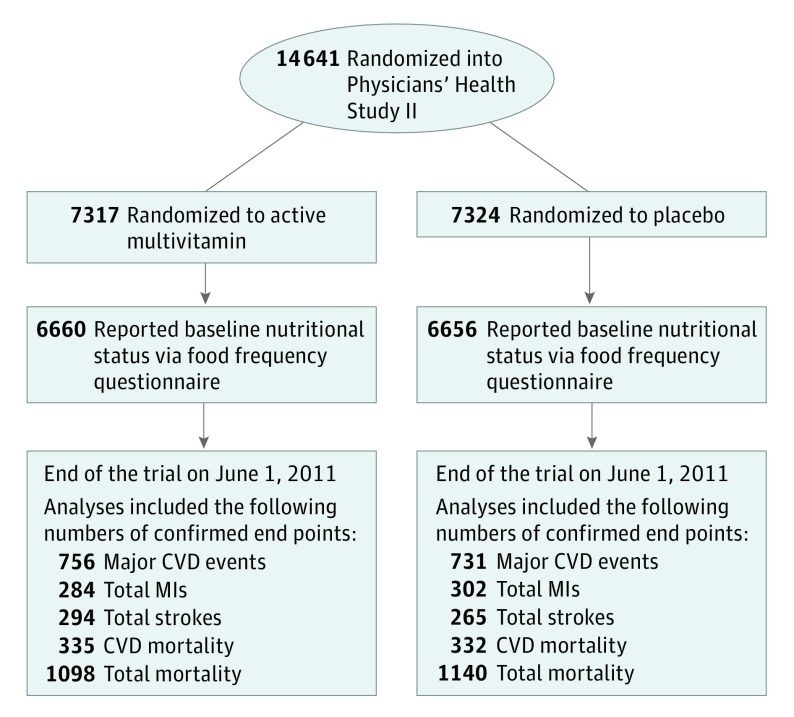
The PHS II recruitment was done in 2 phases. Phase 1 began in July 1997, when 18 763 men from the PHS I without a history of cirrhosis or active liver disease, not taking anticoagulant agents, and not having serious illnesses that may hinder participation were invited to participate. Men also had to be willing to forgo use of multivitamins or individual supplements containing more than 100% of the recommended dietary allowance of vitamin E, vitamin C, beta-carotene, and vitamin A. Men with a history of myocardial infarction (MI), stroke, or cancer remained eligible. Phase 2 began in July 1999, when 254 597 additional US male physicians 50 years or older using the same eligibility criteria as for the PHS I participants were invited to participate. In total, 14 641 men 50 years or older (including 7641 participants from the PHS I and 7000 new participants) were randomized into the PHS II. Before randomization, 754 men (5.1%) had a history of MI or stroke. For the analyses herein, we included 13 316 men (91.0%) who completed a baseline 116-item semiquantitative food frequency questionnaire (FFQ) (Figure). The institutional review board at Brigham and Women’s Hospital approved the trial protocol (Supplement 2), and written informed consent was provided by all participants.
Figure. CONSORT Diagram of Men in the Physicians’ Health Study II.
CONSORT indicates Consolidated Standards of Reporting Trials; CVD, cardiovascular disease; and MIs, myocardial infarctions.
Dietary Factors
Food Groups
The baseline FFQ asked men to report how often they had consumed a wide variety of foods on average during the past year. Nine possible responses, ranging from “never or less than once per month” to “6+ times per day,” were recorded. We calculated the mean daily intake (servings per day) of total fruits and vegetables, whole grains, nuts, dairy products, and red and processed meats. The reproducibility and validity of intake of individual food items has been reported to be reasonable, with correlations ranging from 0.2 to 0.7 for fruits and vegetables, 0.6 to 0.7 for whole grains, 0.4 to 0.9 for dairy products, 0.2 to 0.6 for red meat, and 0.5 for fish.
Nutrient Intake and Supplement Use
Intakes of vitamin B6, vitamin B12, folate, vitamin C, vitamin D, calcium, and magnesium (not taking into account contributions from randomization treatments) were calculated by multiplying the intake frequency by the nutrient content of the specified portion size. All nutrients were energy adjusted using the residual method. Prerandomization use of multivitamins and other vitamin and mineral supplements was also assessed and has been validated in women from the Nurse’s Health Study and in men from the Health Professionals Follow-up Study.
Dietary Patterns
The Alternate Healthy Eating Index (AHEI) is based on 11 components, with a score between 0 and 10 given proportionally to intakes of the following: vegetables, fruits, whole grains, nuts and legumes, long-chain omega-3 fats (docosahexaenoic acid and eicosapentaenoic acid), polyunsaturated fatty acids, moderate alcohol consumption, sugar-sweetened drinks and fruit juices, red and processed meat, trans-fat, and sodium. The AHEI ranges from 0 to 110, with a higher score representing a healthier diet. The Alternate Mediterranean Diet (aMED) Score is based on 9 components, with 1 point given if intake is above the median PHS II intake for vegetables, legumes, fruits, nuts, whole-grain cereals, fish, and the ratio of monounsaturated fatty acids to saturated fatty acids. For red and processed meats, 1 point was given if intake was below the median; for alcohol intake, 1 point was given if intake ranged from 10 to 15 g/d. A higher score represents better adherence to the aMED, ranging from 0 and 9.
CVD End Points and Follow-up
Major cardiovascular events (including nonfatal MI, nonfatal stroke, and CVD mortality) represented a prespecified co-primary end point for the PHS II trial, along with total cancer. Secondary end points included total MI and total stroke; we also considered ischemic stroke, cardiovascular death, and total mortality for these analyses. An end points committee of physicians masked to randomized multivitamin treatment assignment confirmed self-reported end points based on relevant medical records. An MI was confirmed according to the World Health Organization criteria, electrocardiogram criteria, or abnormal concentrations of cardiac enzymes. Stroke was defined as a typical neurological deficit, sudden or rapid in onset, and lasting more than 24 hours. Strokes were also classified according to major subtype (ischemic, hemorrhagic, or unknown), with excellent interobserver agreement (Cohen κ = 0.96). Deaths were identified through reports from family members, postal authorities, and the National Death Index. Follow-up rate of morbidity was 98.2% and follow-up rate of mortality was 99.9%.
Statistical Analysis
Primary analyses were based on the intention-to-treat principle. We used Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% CIs of CVD, which were stratified on the presence of CVD at randomization and adjusted for age (in years), PHS cohort (original PHS I participant or new PHS II participant), and randomized vitamin E, vitamin C, and beta-carotene assignments.
To investigate effect modification of randomized multivitamin treatment on CVD events by baseline nutritional status, we computed HRs of CVD for multivitamin use stratified by tertiles of intake for food groups, individual nutrients, and dietary patterns, including the AHEI and aMED Score. We also investigated effect modification by baseline dietary patterns by restricting our analyses to men 70 years or older and to men compliant with the multivitamin intervention, defined as taking at least two-thirds of the study pills.
A global test for interaction between randomized multivitamin treatment and each baseline nutritional factor was performed by computing a Wald statistic. All statistical analyses were conducted with a software program (SAS, version 9.3; SAS Institute Inc).
Results
Baseline Nutritional Characteristics
In total, 13 316 male physicians were observed for a mean (SD) follow-up of 11.4 (2.3) years. In Table 1, baseline nutritional factors are summarized according to randomized multivitamin treatment assignment, and there were no significant differences in intakes observed comparing the placebo group vs the active multivitamin group. Adherence to active multivitamin and its respective placebo was 76.8% and 77.1% at 4 years (P = .71), 72.3% and 70.7% at 8 years (P = .15), and 67.5% and 67.1% at the end of follow-up (P = .70).
Table 1. Self-reported Baseline Nutritional Characteristics According to Multivitamin Treatment Assignment in 13 316 Men From the Physicians’ Health Study II (PHS II)a.
| Variable | Placebo | Active Multivitamin | P Value |
|---|---|---|---|
| Age at PHS II randomization, mean (SD), y | 64.0 (9.1) | 64.0 (9.0) | .98 |
| Food Groups, Mean (SD) | |||
| Fruits and vegetables, servings/d | 4.7 (2.6) | 4.7 (2.6) | .91 |
| Whole grains, g/d | 28.3 (22.5) | 28.1 (21.9) | .52 |
| Nuts, servings/d | 0.4 (0.8) | 0.4 (0.8) | .41 |
| Dairy products, servings/d | 2.2 (1.7) | 2.3 (1.7) | .14 |
| Red and processed meats, servings/d | 0.7 (0.6) | 0.7 (0.6) | .61 |
| Nutrient Intake, Mean (SD) | |||
| Vitamin B6, mg/d | 5.3 (17.8) | 4.8 (16.6) | .16 |
| Vitamin B12, µg/d | 9.4 (13.1) | 9.2 (11.6) | .30 |
| Folate, µg/d | 699.0 (490.0) | 689.6 (479.6) | .28 |
| Vitamin C, mg/d | 199.8 (235.9) | 192.1 (222.6) | .06 |
| Vitamin D, IU/d | 232.6 (196.3) | 233.1 (198.4) | .89 |
| Calcium, mg/d | 780.3 (394.6) | 784.6 (402.2) | .54 |
| Magnesium, mg/d | 305.2 (69.1) | 305.0 (69.3) | .91 |
| Dietary Patterns, Mean (SD) | |||
| Alternate Healthy Eating Index | 43.6 (10.6) | 43.6 (10.6) | .89 |
| Alternate Mediterranean Diet Score | 3.9 (1.8) | 3.9 (1.8) | .65 |
| Prerandomization Supplement Use, No./Total No. (%) | |||
| Multivitamin use | 1680/6655 (25.2) | 1699/6659 (25.5) | .74 |
| ≥2 Supplements simultaneously | 509/6656 (7.6) | 533/6660 (8.0) | .46 |
The distributions of baseline characteristics in the placebo and active multivitamin groups were compared using 2-sample t tests for means and χ2 tests for proportions.
Main PHS II Trial Findings
In previously published main PHS II trial analyses, randomization to daily multivitamin use had no effect on the primary end point of major cardiovascular events (HR, 1.01; 95% CI, 0.91-1.10) and the secondary end points of total MI (HR, 0.93; 95% CI, 0.80-1.09) and total stroke (HR, 1.06; 95% CI, 0.91-1.23). Furthermore, multivitamin use was not statistically significantly associated with total mortality (HR, 0.94; 95% CI, 0.88-1.02) or CVD mortality (HR, 0.95; 95% CI, 0.83-1.09).
Effect Modification by Baseline Food Intake
In Table 2, we summarize results for the effect of multivitamin use on major CVD end points and total mortality stratified by baseline intake of representative food groups. There was no evidence of any statistically significant interaction between multivitamin use and any of the food groups on risk of CVD mortality and total mortality.
Table 2. Multivitamin Use and Risk of Major Cardiovascular Disease (CVD) Events and Total Mortality by Categories of Baseline Food Intake.
| Variable | No. | HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Major CVD Eventsa | Total MIs | Total Strokes | Ischemic Strokes | CVD Mortality | Total Mortality | ||
| Fruits and Vegetables, Servings/db | |||||||
| <3.4 | 4438 | 1.11 (0.92-1.33) |
1.04 (0.78-1.39) |
1.26 (0.93-1.69) |
1.23 (0.89-1.70) |
0.98 (0.74-1.30) |
0.99 (0.85-1.15) |
| 3.4 to <5.1 | 4435 | 1.08 (0.90-1.29) |
1.02 (0.77-1.35) |
1.14 (0.86-1.52) |
1.20 (0.88-1.64) |
0.99 (0.75-1.30) |
0.91 (0.78-1.05) |
| ≥5.1 | 4437 | 0.89 (0.75-1.05) |
0.78 (0.59-1.03) |
0.93 (0.70-1.23) |
0.92 (0.67-1.26) |
1.05 (0.82-1.33) |
0.98 (0.86-1.12) |
| P value for interaction | NA | .15 | .28 | .34 | .36 | .92 | .68 |
| Whole Grains, g/db | |||||||
| <15.3 | 4219 | 1.02 (0.85-1.22) |
0.81 (0.61-1.08) |
1.01 (0.75-1.34) |
1.14 (0.83-1.57) |
1.05 (0.81-1.36) |
0.93 (0.81-1.07) |
| 15.3 to <32.7 | 4220 | 1.00 (0.83-1.20) |
1.01 (0.75-1.36) |
1.11 (0.81-1.51) |
1.08 (0.77-1.52) |
0.90 (0.68-1.19) |
0.92 (0.79-1.07) |
| ≥32.7 | 4219 | 1.07 (0.90-1.28) |
1.01 (0.76-1.33) |
1.23 (0.92-1.65) |
1.17 (0.85-1.60) |
1.12 (0.85-1.47) |
1.05 (0.90-1.22) |
| P value for interaction | NA | .85 | .48 | .62 | .95 | .52 | .39 |
| Nuts, Servings/db | |||||||
| <0.07 | 3464 | 1.08 (0.89-1.31) |
0.91 (0.65-1.28) |
1.25 (0.92-1.69) |
1.33 (0.95-1.85) |
1.02 (0.77-1.35) |
1.00 (0.86-1.16) |
| 0.07 to <0.14 | 5743 | 1.02 (0.87-1.20) |
0.84 (0.65-1.08) |
1.19 (0.91-1.56) |
1.18 (0.88-1.58) |
1.11 (0.87-1.43) |
1.05 (0.92-1.21) |
| ≥0.14 | 3918 | 0.93 (0.77-1.12) |
1.12 (0.84-1.48) |
0.81 (0.60-1.11) |
0.78 (0.55-1.10) |
0.82 (0.62-1.08) |
0.83 (0.71-0.96) |
| P value for interaction | NA | .54 | .32 | .10 | .07 | .27 | .06 |
| Dairy Products, Servings/db | |||||||
| <1.4 | 4433 | 1.09 (0.90-1.32) |
0.88 (0.65-1.19) |
1.32 (0.96-1.80) |
1.28 (0.92-1.80) |
1.15 (0.85-1.57) |
0.99 (0.85-1.17) |
| 1.4 to <2.4 | 4410 | 1.13 (0.95-1.34) |
1.19 (0.90-1.58) |
1.10 (0.83-1.46) |
1.07 (0.79-1.46) |
1.10 (0.85-1.43) |
0.91 (0.78-1.05) |
| ≥2.4 | 4440 | 0.86 (0.73-1.02) |
0.79 (0.60-1.02) |
0.95 (0.73-1.26) |
1.03 (0.76-1.40) |
0.84 (0.66-1.07) |
0.97 (0.85-1.11) |
| P value for interaction | NA | .06 | .16 | .32 | .61 | .19 | .66 |
| Red and Processed Meats, Servings/db | |||||||
| <0.4 | 4334 | 0.95 (0.79-1.15) |
0.87 (0.64-1.18) |
1.11 (0.82-1.50) |
1.05 (0.75-1.47) |
0.91 (0.68-1.23) |
0.90 (0.77-1.06) |
| 0.4 to <0.9 | 4565 | 0.99 (0.83-1.18) |
1.04 (0.79-1.37) |
0.99 (0.74-1.33) |
1.05 (0.76-1.43) |
0.85 (0.65-1.10) |
0.91 (0.79-1.06) |
| ≥0.9 | 4369 | 1.08 (0.92-1.28) |
0.89 (0.68-1.17) |
1.18 (0.90-1.55) |
1.22 (0.90-1.65) |
1.24 (0.97-1.58) |
1.05 (0.92-1.19) |
| P value for interaction | NA | .59 | .64 | .70 | .72 | .09 | .26 |
Abbreviations: HR, hazard ratio; MIs, myocardial infarctions; NA, not applicable.
Adjusted for age, Physicians’ Health Study cohort (original PHS I participant or new PHS II participant), and randomized treatment assignment (beta-carotene, vitamin E, and vitamin C).
We excluded men with a total energy intake outside of the range of 600 to 3500 kcal/d.
Effect Modification by Baseline Nutrient Intake
In Table 3, we summarize results of the effect of multivitamin use on CVD and mortality by baseline nutrient intake, with no consistent patterns by either specific nutrient. Most interactions tested revealed no effect measure modification by baseline nutrient intake, with a few potential exceptions that are more likely explained by chance and multiple testing. There were statistically significant interaction effects between multivitamin use and vitamin B12 intake on CVD mortality (P = .04 for interaction) and total mortality (P = .04 for interaction), but with inconsistent patterns in HRs across tertiles of vitamin B12 intake. We also found a statistically significant interaction effect between multivitamin use and vitamin B6 intake on risk of MI (P = .01 for interaction). Finally, there was a statistically significant interaction between multivitamin use and vitamin D intake on CVD mortality (P = .03 for interaction).
Table 3. Multivitamin Use and Risk of Major Cardiovascular Disease (CVD) Events and Total Mortality by Categories of Baseline Nutrient Intake.
| Variable | No. | HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Major CVD Eventsa | Total MIs | Total Strokes | Ischemic Strokes | CVD Mortality | Total Mortality | ||
| Vitamin B6, mg/db | |||||||
| <1.86 | 4219 | 1.21 (1.00-1.47) |
1.36 (1.00-1.85) |
1.20 (0.87-1.66) |
1.40 (0.97-2.01) |
0.95 (0.71-1.26) |
0.97 (0.83-1.13) |
| 1.86 to <2.36 | 4220 | 1.01 (0.85-1.22) |
0.86 (0.64-1.15) |
1.06 (0.79-1.40) |
0.97 (0.71-1.32) |
1.17 (0.89-1.56) |
0.97 (0.83-1.13) |
| ≥2.36 | 4219 | 0.92 (0.77-1.09) |
0.74 (0.56-0.98) |
1.11 (0.84-1.47) |
1.14 (0.84-1.55) |
0.97 (0.75-1.25) |
0.96 (0.84-1.10) |
| P value for interaction | NA | .11 | .01 | .85 | .33 | .50 | .99 |
| Vitamin B12, µg/db | |||||||
| <4.76 | 4219 | 1.12 (0.92-1.37) |
1.14 (0.84-1.55) |
1.21 (0.87-1.67) |
1.32 (0.92-1.88) |
0.83 (0.60-1.15) |
0.85 (0.71-1.01) |
| 4.76 to <7.94 | 4220 | 1.08 (0.91-1.30) |
0.95 (0.71-1.27) |
1.08 (0.82-1.44) |
1.00 (0.73-1.37) |
1.36 (1.03-1.79) |
1.13 (0.97-1.31) |
| ≥7.94 | 4219 | 0.93 (0.78-1.10) |
0.79 (0.60-1.04) |
1.08 (0.81-1.42) |
1.14 (0.84-1.55) |
0.93 (0.73-1.18) |
0.93 (0.81-1.06) |
| P value for interaction | NA | .29 | .21 | .86 | .53 | .04 | .04 |
| Folate, µg/db | |||||||
| <427.5 | 4219 | 1.11 (0.92-1.34) |
1.04 (0.78-1.40) |
1.08 (0.81-1.45) |
1.11 (0.80-1.53) |
1.13 (0.85-1.49) |
1.03 (0.89-1.20) |
| 427.5 to <512.9 | 4220 | 1.10 (0.91-1.32) |
1.01 (0.75-1.36) |
1.18 (0.87-1.61) |
1.24 (0.90-1.72) |
1.10 (0.84-1.44) |
0.90 (0.77-1.05) |
| ≥512.9 | 4219 | 0.90 (0.76-1.08) |
0.80 (0.61-1.05) |
1.07 (0.80-1.43) |
1.06 (0.77-1.46) |
0.87 (0.67-1.14) |
0.97 (0.84-1.12) |
| P value for interaction | NA | .19 | .36 | .88 | .78 | .36 | .47 |
| Vitamin C, mg/db | |||||||
| <107.3 | 4219 | 1.13 (0.93-1.37) |
0.97 (0.73-1.30) |
1.07 (0.78-1.45) |
1.16 (0.82-1.63) |
1.25 (0.92-1.68) |
1.00 (0.86-1.18) |
| 107.3 to <175.4 | 4220 | 1.07 (0.90-1.29) |
1.03 (0.76-1.39) |
1.23 (0.92-1.64) |
1.20 (0.87-1.65) |
0.97 (0.75-1.26) |
0.94 (0.82-1.09) |
| ≥175.4 | 4219 | 0.91 (0.77-1.09) |
0.83 (0.63-1.10) |
1.04 (0.78-1.39) |
1.05 (0.77-1.44) |
0.93 (0.72-1.21) |
0.96 (0.83-1.11) |
| P value for interaction | NA | .23 | .56 | .70 | .84 | .31 | .85 |
| Vitamin D, IU/db | |||||||
| <126.1 | 4219 | 1.12 (0.91-1.37) |
1.03 (0.75-1.40) |
1.34 (0.96-1.89) |
1.50 (1.02-2.19) |
0.97 (0.71-1.33) |
0.89 (0.75-1.05) |
| 126.1 to <217.4 | 4220 | 1.14 (0.95-1.36) |
0.99 (0.74-1.31) |
1.07 (0.81-1.41) |
1.06 (0.78-1.43) |
1.34 (1.03-1.74) |
1.04 (0.90-1.21) |
| ≥217.4 | 4219 | 0.88 (0.74-1.04) |
0.83 (0.63-1.09) |
1.02 (0.77-1.34) |
1.01 (0.75-1.37) |
0.82 (0.64-1.06) |
0.96 (0.83-1.10) |
| P value for interaction | NA | .07 | .54 | .44 | .25 | .03 | .37 |
| Calcium, mg/db | |||||||
| <573.8 | 4219 | 1.03 (0.85-1.26) |
1.02 (0.76-1.38) |
1.13 (0.81-1.57) |
1.19 (0.83-1.71) |
0.86 (0.64-1.17) |
0.86 (0.73-1.01) |
| 573.8 to <816.1 | 4220 | 1.08 (0.90-1.29) |
0.77 (0.58-1.03) |
1.29 (0.96-1.74) |
1.19 (0.86-1.63) |
1.32 (1.01-1.72) |
1.04 (0.89-1.20) |
| ≥816.1 | 4219 | 0.98 (0.82-1.16) |
1.05 (0.79-1.38) |
0.97 (0.74-1.26) |
1.05 (0.77-1.41) |
0.91 (0.71-1.18) |
0.99 (0.87-1.14) |
| P value for interaction | NA | .72 | .27 | .36 | .81 | .07 | .22 |
| Magnesium, mg/db | |||||||
| <270.1 | 4219 | 1.11 (0.93-1.34) |
1.09 (0.81-1.47) |
1.17 (0.87-1.58) |
1.27 (0.91-1.78) |
0.91 (0.70-1.20) |
0.87 (0.75-1.01) |
| 270.1 to <322 | 4220 | 1.00 (0.83-1.21) |
0.97 (0.73-1.29) |
1.06 (0.79-1.43) |
1.06 (0.77-1.46) |
1.21 (0.92-1.61) |
1.06 (0.91-1.24) |
| ≥322 | 4219 | 0.98 (0.82-1.17) |
0.78 (0.59-1.04) |
1.11 (0.83-1.47) |
1.09 (0.79-1.50) |
0.97 (0.75-1.27) |
0.99 (0.85-1.14) |
| P value for interaction | NA | .56 | .27 | .91 | .70 | .33 | .18 |
Abbreviations: HR, hazard ratio; MIs, myocardial infarctions; NA, not applicable.
Adjusted for age, Physicians’ Health Study cohort (original PHS I participant or new PHS II participant), and randomized treatment assignment (beta-carotene, vitamin E, and vitamin C).
We excluded men with a total energy intake outside of the range of 600 to 3500 kcal/d.
Effect Modification by Baseline Dietary Patterns
In Table 4, we summarize results for effect modification by baseline dietary patterns as represented by the AHEI and aMED Score. We found no evidence that the effect of multivitamin use was modified by either dietary pattern (P > .05 for interaction for all). Men in the highest tertile of the aMED Score (score of 5-9) randomized to receive the active multivitamin had a nonsignificant reduction in MI.
Table 4. Multivitamin Use and Risk of Major Cardiovascular Disease (CVD) Events and Total Mortality by Categories of Baseline Dietary Patterns.
| Variable | No. | HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Major CVD Eventsa | Total MIs | Total Strokes | Ischemic Strokes | CVD Mortality | Total Mortality | ||
| Alternate Healthy Eating Indexb | |||||||
| 15.5 to <40.5 | 3391 | 1.15 (0.94-1.41) |
1.09 (0.80-1.50) |
1.34 (0.97-1.84) |
1.36 (0.96-1.92) |
0.96 (0.70-1.32) |
0.95 (0.81-1.13) |
| 40.5 to <49.5 | 3398 | 0.99 (0.80-1.21) |
0.78 (0.57-1.06) |
1.10 (0.78-1.56) |
1.11 (0.77-1.62) |
1.15 (0.84-1.58) |
0.95 (0.80-1.13) |
| 49.5 to 82.5 | 3606 | 0.96 (0.77-1.19) |
0.88 (0.62-1.24) |
0.98 (0.68-1.40) |
0.90 (0.60-1.36) |
0.97 (0.69-1.35) |
1.04 (0.86-1.25) |
| P value for interaction | NA | .40 | .32 | .43 | .33 | .68 | .74 |
| Alternate Mediterranean Diet Scoreb | |||||||
| 0-2 | 3275 | 1.06 (0.86-1.30) |
1.06 (0.76-1.48) |
1.13 (0.81-1.57) |
1.13 (0.80-1.60) |
0.94 (0.69-1.27) |
0.88 (0.75-1.03) |
| 3-4 | 5114 | 1.02 (0.86-1.20) |
1.03 (0.79-1.34) |
1.04 (0.80-1.35) |
1.07 (0.80-1.43) |
0.99 (0.77-1.27) |
1.05 (0.92-1.20) |
| 5-9 | 4927 | 0.97 (0.82-1.15) |
0.77 (0.59-1.00) |
1.12 (0.85-1.49) |
1.13 (0.82-1.56) |
1.05 (0.82-1.35) |
0.91 (0.79-1.05) |
| P value for interaction | NA | .81 | .21 | .89 | .96 | .84 | .19 |
Abbreviations: HR, hazard ratio; MIs, myocardial infarctions; NA, not applicable.
Adjusted for age, Physicians’ Health Study cohort (original PHS I participant or new PHS II participant), and randomized treatment assignment (beta-carotene, vitamin E, and vitamin C).
We excluded men with a total energy intake outside of the range of 600 to 3500 kcal/d or missing information on included food items.
We further investigated whether dietary patterns modified the effect of multivitamin use among 3583 men 70 years or older. In these analyses, men receiving the active multivitamin had a statistically significant reduction in MI if they were in the second tertile of the AHEI (HR for active multivitamin vs placebo, 0.51, 95% CI, 0.32-0.82 or in the highest tertile of the aMED Score (HR for active multivitamin vs placebo, 0.55; 95% CI, 0.37-0.81).
We performed sensitivity analyses restricted to men who were adherent to the active multivitamin or placebo, defined as taking at least two-thirds of the study pills. We found no evidence that our results were affected by compliance with the multivitamin intervention.
Effect Modification by Baseline Dietary Supplement Use
We also investigated whether the effect of multivitamin use and risk of major CVD and total mortality was modified by prerandomization use of multivitamins, vitamin E supplements, and total number of dietary supplements taken. We found no evidence of effect modification.
Sensitivity Analyses
When categorizing men into quartiles by intake of food groups, nutrients, and dietary patterns, we still generally found no overall evidence of a potential modifying role of baseline nutritional status. However, there was a statistically significant interaction effect between multivitamin use and vitamin D intake on total mortality (P = .04 for interaction), with nonsignificant lower mortality in the lowest quartile of vitamin D intake (HR, 0.83; 95% CI, 0.68-1.01) and nonsignificant higher mortality in the third quartile of vitamin D intake (HR, 1.18; 95% CI, 0.99-1.39).
Discussion
In this randomized clinical trial of daily multivitamin use in middle-aged and older men that lasted more than a decade, there was no evidence that baseline nutritional status modified the effect of multivitamin use on major CVD events and total mortality. Inconsistent findings were observed for selected dietary factors and end points that are likely explained by multiple testing. The PHS II included physician participants, who had on average a healthier diet than the general population. Therefore, the generalizability of these results to populations with different dietary patterns is subject to caution.
Given the persistent and common use of multivitamins in older US adults, it is important to understand the long-term effects of multivitamin use and how they may be influenced by baseline nutritional status. Whereas individual and limited combinations of vitamins and minerals were previously tested at much higher amounts than can be achieved through diet alone, a multivitamin more closely approximates nutritional intake that may be achieved via a well-balanced, heart-healthy diet.
Men who have lower dietary vitamin and mineral intake may be expected to benefit more from taking a multivitamin compared with men who already have sufficient micronutrient intake. This practice would most likely extend to averting nutritional insufficiency or deficiency, but it has been unclear whether improvements in nutritional status extend to reductions in CVD and other major morbidity. Our results suggest that multivitamin use among men with lower intake of major dietary factors did not lower risk of CVD or total mortality. However, the PHS II included male physicians, who were on average likely better nourished than the general American population. They had higher mean intakes of fruits, vegetables, whole grains, and milk and cheese compared with men from the nationally representative National Health and Examination Survey. Therefore, we may not have had the required ranges or extremes of dietary intake to sufficiently distinguish whether particular subgroups of men may benefit more or less from multivitamin use based on baseline nutritional status. Optimally, the evaluation of nutritional biomarkers would provide a more objective assessment of nutritional status and complement our findings.
Another consideration is multivitamin use in older men, in whom pathophysiological changes may affect nutrient absorption and subsequent nutritional status, altering vitamin and mineral requirements. Diet alone may not be enough to meet these changes in nutritional requirements. For example, vitamin B12 deficiency is highly prevalent among adults 65 years or older because of poor diet and diminished absorption associated with age. However, in our analyses, men 70 years or older who were in the highest tertiles of the AHEI and more adherent to the aMED had reductions in MI with multivitamin use.
To date, the PHS II remains the only completed, long-term randomized clinical trial that has tested a commonly used multivitamin supplement with amounts of all essential vitamins and minerals to help meet recommended daily intakes or dietary allowances. Moreover, to our knowledge, this trial is the first study that has investigated whether baseline nutritional status modifies the effect of multivitamin use on risk of CVD. Previous clinical trials that investigated the modifying role of baseline nutritional status on CVD risk were limited to individual or selected combinations of vitamins and minerals at much higher amounts than were found in the multivitamin tested in the PHS II. Furthermore, most of these trials were conducted among higher-risk populations, making comparisons across studies difficult. The Women’s Antioxidant and Folic Acid Cardiovascular Study tested a combination of folic acid (2.5 mg/d), vitamin B6 (50 mg/d), and vitamin B12 (1 mg/d) among female health professionals at higher risk of CVD, with no evidence of effect modification by baseline folate intake or multivitamin use. In contrast, the China Stroke Primary Prevention Trial tested a folic acid supplement (0.8 mg/d) among hypertensive women and men and reported stronger reductions in stroke among participants with the lowest baseline folate blood concentrations (<5.6 ng/mL) (to convert folate level to nanomoles per liter, multiply by 2.266).
Only a few prospective cohort studies have examined if an association between multivitamin use and CVD risk is modified by baseline dietary intake. In the Women’s Health Study, the association between multivitamin use and risk of CVD was modified by age and fruit and vegetable intake, with women who were older or who had low fruit and vegetable intake having a lower risk. Moreover, a prospective cohort study of Swedish men demonstrated that dietary supplement use was associated with lower CVD mortality only among men with an inadequate diet. These results highlight the importance of additional research on the role of baseline nutritional status when investigating the use of multivitamins (and other dietary supplements) in CVD prevention.
Strengths and Limitations
Our study has several strengths. The PHS II was a large-scale, long-term randomized clinical trial that tested a commonly used multivitamin, with high compliance and long follow-up. Randomization of multivitamin use eliminates any potential confounding by overall healthy behavioral factors, which typically limits findings from observational studies. Moreover, we had data on a wide range of dietary factors to comprehensively investigate the potential modifying role of baseline nutritional status. However, several important limitations should be considered. Dietary intake was self-reported via an FFQ; therefore, measurement error may have influenced our results. Dietary status was only assessed at baseline; different effects may have been observed if changes in diet had been taken into account. The multivitamin formulation used has changed incrementally over the years; for example, vitamin D content is higher in current formulations than in that tested throughout the PHS II trial period. However, the formulations are similar overall, and any small differences are unlikely to affect the generalizability of our findings to a high extent. Concomitant medication use for treating CVD risk factors, such as hypercholesterolemia, hypertension, and type 2 diabetes, may also have influenced our results. However, because of randomization, these factors were evenly distributed across multivitamin treatment arms, and there was no evidence of effect modification by these factors at baseline. Moreover, because we performed multiple tests across numerous dietary factors, we cannot exclude the possibility that some statistically significant effects were false-positive results.
Conclusions
In this randomized clinical trial of middle-aged and older men, we found that long-term daily multivitamin use does not prevent major CVD events, regardless of baseline nutritional status. Future studies should seek to include a larger proportion of individuals with a wider range of dietary intake and should use nutritional biomarkers as more objective assessments of baseline nutritional status that account for differences in absorption and metabolism to understand how they influence the long-term effects of multivitamin use on CVD risk. The recently initiated Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) trial, which is testing a multivitamin supplement with 4 years of treatment and follow-up, will provide further context of its role in CVD prevention among older women and older men, including potential effect modification by baseline nutritional status.
eTable. Vitamins and Minerals Contained in the Centrum Silver Formulation Used in the Physicians’ Health Study II Trial
Trial Protocol
References
- 1.Bailey RL, Gahche JJ, Lentino CV, et al. . Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141(2):261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355-361. [DOI] [PubMed] [Google Scholar]
- 3.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824-834. [DOI] [PubMed] [Google Scholar]
- 4.Rautiainen S, Akesson A, Levitan EB, Morgenstern R, Mittleman MA, Wolk A. Multivitamin use and the risk of myocardial infarction: a population-based cohort of Swedish women. Am J Clin Nutr. 2010;92(5):1251-1256. [DOI] [PubMed] [Google Scholar]
- 5.Watkins ML, Erickson JD, Thun MJ, Mulinare J, Heath CW Jr. Multivitamin use and mortality in a large prospective study. Am J Epidemiol. 2000;152(2):149-162. [DOI] [PubMed] [Google Scholar]
- 6.Rimm EB, Willett WC, Hu FB, et al. . Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279(5):359-364. [DOI] [PubMed] [Google Scholar]
- 7.Rautiainen S, Rist PM, Glynn RJ, Buring JE, Gaziano JM, Sesso HD. Multivitamin use and the risk of cardiovascular disease in men. J Nutr. 2016;146(6):1235-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmquist C, Larsson S, Wolk A, de Faire U. Multivitamin supplements are inversely associated with risk of myocardial infarction in men and women: Stockholm Heart Epidemiology Program (SHEEP). J Nutr. 2003;133(8):2650-2654. [DOI] [PubMed] [Google Scholar]
- 9.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. . Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch Intern Med. 2009;169(3):294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR Jr. Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med. 2011;171(18):1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160(4):339-349. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Jensen CD, Norkus EP, et al. . Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: a cross-sectional study. Nutr J. 2007;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Kim J. Factors affecting the use of dietary supplements by Korean adults: data from the Korean National Health and Nutrition Examination Survey III. J Am Diet Assoc. 2009;109(9):1599-1605. [DOI] [PubMed] [Google Scholar]
- 14.Robson PJ, Siou GL, Ullman R, Bryant HE. Sociodemographic, health and lifestyle characteristics reported by discrete groups of adult dietary supplement users in Alberta, Canada: findings from The Tomorrow Project. Public Health Nutr. 2008;11(12):1238-1247. [DOI] [PubMed] [Google Scholar]
- 15.Reedy J, Haines PS, Campbell MK. Differences in fruit and vegetable intake among categories of dietary supplement users. J Am Diet Assoc. 2005;105(11):1749-1756. [DOI] [PubMed] [Google Scholar]
- 16.Sesso HD, Christen WG, Bubes V, et al. . Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaziano JM, Sesso HD, Christen WG, et al. . Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christen WG, Glynn RJ, Manson JE, et al. . Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121(2):525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125-134. [DOI] [PubMed] [Google Scholar]
- 20.Sesso HD, Buring JE, Christen WG, et al. . Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. . Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858-867. [DOI] [PubMed] [Google Scholar]
- 22.Agricultural Research Service. Composition of Foods: Raw, Processed, Prepared Washington, DC: US Dept of Agriculture; 1993. Agriculture Handbook 8, 1992 Supplement. [Google Scholar]
- 23.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17-27. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, et al. . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. [DOI] [PubMed] [Google Scholar]
- 26.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofman A, Ott A, Breteler MM, et al. . Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151-154. [DOI] [PubMed] [Google Scholar]
- 28.Atiya M, Kurth T, Berger K, Buring JE, Kase CS; Women’s Health Study . Interobserver agreement in the classification of stroke in the Women’s Health Study. Stroke. 2003;34(2):565-567. [DOI] [PubMed] [Google Scholar]
- 29.Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention - a global overview. Nat Rev Endocrinol. 2016;12(7):407-420. [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute, Applied Research Program, Risk Factor Monitoring and Methods. Usual dietary intakes: food intakes, US population, 2001-2004. https://epi.grants.cancer.gov/diet/usualintakes/pop/2001-04. Updated May 6, 2016. Accessed 2017.
- 31.Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta-analysis. Ageing Res Rev. 2013;12(2):561-566. [DOI] [PubMed] [Google Scholar]
- 32.Stover PJ. Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care. 2010;13(1):24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert CM, Cook NR, Gaziano JM, et al. . Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo Y, Li J, Qin X, et al. ; CSPPT Investigators . Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325-1335. [DOI] [PubMed] [Google Scholar]
- 35.Rautiainen S, Lee IM, Rist PM, et al. . Multivitamin use and cardiovascular disease in a prospective study of women. Am J Clin Nutr. 2015;101(1):144-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messerer M, Håkansson N, Wolk A, Akesson A. Dietary supplement use and mortality in a cohort of Swedish men. Br J Nutr. 2008;99(3):626-631. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. Cocoa Supplement and Multivitamin Outcomes Study (COSMOS). NCT02422745. https://clinicaltrials.gov/ct2/show/NCT02422745. Updated November 10, 2016. Accessed February 18, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Vitamins and Minerals Contained in the Centrum Silver Formulation Used in the Physicians’ Health Study II Trial
Trial Protocol