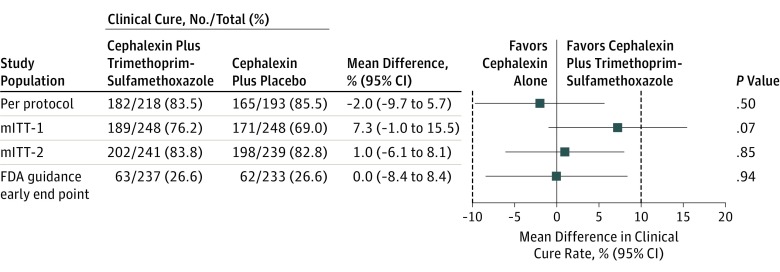
Figure 2. Clinical Cure Rates Among Participants With Cellulitis Treated With Cephalexin Plus Trimethoprim-Sulfamethoxazole or Cephalexin Plus Placebo in the Modified Intention-to-Treat, Per-Protocol, and FDA Guidance Early End-Point Populations.
See Table 1 for a description of the per-protocol, modified intention-to-treat (mITT-1 and mITT-2), and US Food and Drug Administration (FDA) guidance early end-point (response rate reported) populations and outcome definitions. Dashed lines indicate the 10% predetermined threshold of clinically significant difference.