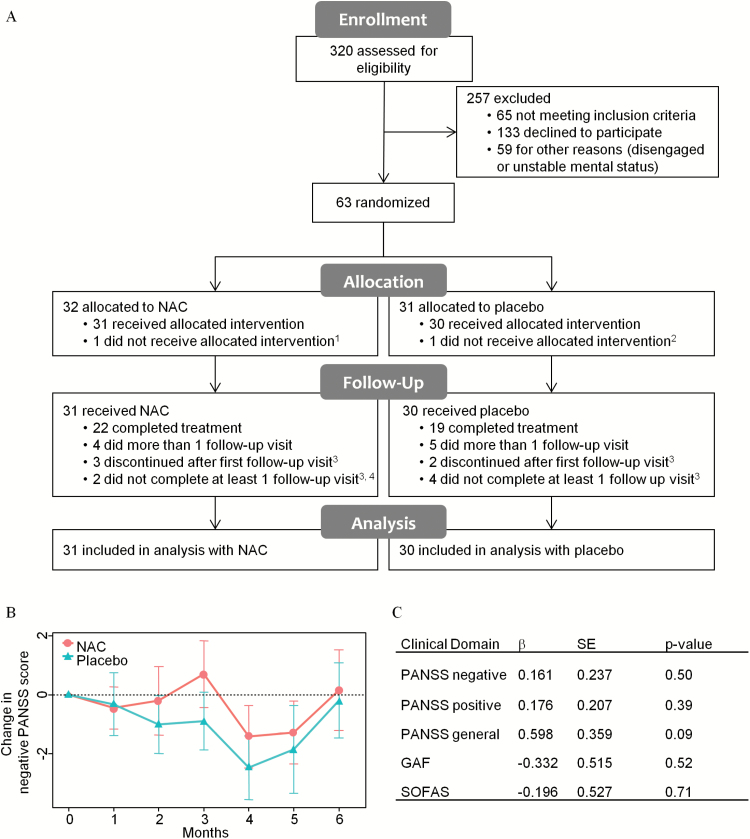
Fig. 1.
CONSORT flow diagram, primary and secondary outcomes. (A) Reasons for withdrawal from trial, 1white matter lesions detected at baseline MRI scan; 2withdrew at baseline; 3lack of motivation; 4side effects. (B) Change in PANSS− score vs baseline in NAC (red circles) and placebo (green triangles) groups at each visit. (C) Comparison of NAC vs placebo in the rate of improvement. PANSS, Positive and Negative Syndrome Scale; GAF, General Assessment of Functioning; SOFAS, Social and Occupational Functioning Assessment Scale; β, an estimate of NAC effect vs placebo; SE, standard error.