Abstract
Background: Schizophrenia is associated with increased physical morbidity and early mortality, suggesting that the aging process may be accelerated in schizophrenia. However, the biological underpinnings of these alterations in aging in schizophrenia are unclear. Method: We conducted a detailed search of peer-reviewed empirical studies to evaluate evidence for accelerated biological aging in schizophrenia based on systemic, age-related biomarkers. We included studies that investigated differences between persons with schizophrenia and healthy comparison subjects in levels of biomarkers known to be associated with aging and examined the relationship of these biomarkers to age in the 2 groups. Results: Forty-two articles that met our selection criteria were reviewed. Nearly 75% reported abnormal biomarker levels among individuals with schizophrenia, including indices of inflammation, cytotoxicity, oxidative stress, metabolic health, gene expression, and receptor/synaptic function, with medium to large effect sizes reported in many studies. Twenty-nine percent of the studies observed differential age-related decline in schizophrenia. Markers of receptor/synaptic function and gene expression were most frequently differentially related to age in schizophrenia. Schizophrenia patients with greater disease severity and longer illness duration exhibited higher levels of inflammatory and oxidative stress biomarkers and shorter telomere length. Conclusions: Most studies show biomarker abnormalities in schizophrenia, and there is some suggestion for accelerated aging. Although definitive interpretation is limited by cross-sectional design of the published reports, findings in the area of gene expression and synaptic function are promising and pave the way for future longitudinal studies needed to fully test this hypothesis.
Keywords: psychosis, mortality, inflammation, cytotoxicity, oxidative stress
Introduction
“Besides the psychic disorders, there are also in the physical domain a series of morbid phenomena to record… The obscurity… has been a frequent motive for an examination of the blood picture and of metabolism, but the findings up to now are not very satisfactory.”
—Emil Kraepelin, 19131
It has been known for more than a century1 that schizophrenia is associated with markedly increased physical comorbidity and mortality. Physiological changes seen throughout the body with normal aging occur at an earlier age in people with schizophrenia than in healthy comparison subjects (HCs).2 Younger adults with schizophrenia are prone to diseases associated with aging such as diabetes and cardiovascular disorders.3–7 The average life span of a person with schizophrenia is 15–20 years shorter than that of an unaffected person,2,8 and patients with schizophrenia have 2–12 times higher mortality rate than age-comparable general population.9–11 Given that lifespans are generally increasing for the population as a whole,12 understanding alterations in the aging process within schizophrenia is clearly imperative.
Two-thirds of the excess deaths in schizophrenia are not from suicide, but due to “natural causes” such as cardiac and metabolic disorders.11 This has led to a provocative suggestion that schizophrenia is not only a brain disease but rather, a disease of the whole body.2,13 Prior attempts to address systemic biological defects in schizophrenia failed,1 at least in part, because of a lack of specific and sensitive systemic biomarkers of aging. Thus, one way to test the hypothesis of accelerated biological aging would be through research on systemic biomarkers of aging such as those associated with immune function, oxidative stress, metabolic function, or cytotoxicity.
Several prior reviews have examined accelerated aging in schizophrenia from various perspectives, including metabolic disease,2,13 cognition,2,13 brain structure,14–16 telomere length (TL),17–19 and oxidative stress.20 However, no publication, to our knowledge, has provided a comprehensive review of multiple biomarkers relevant to the hypothesis of accelerated biological aging in schizophrenia. Although many comparisons and similarities have been drawn between schizophrenia and normal aging, we have a limited understanding as to which biomarkers are most altered with age in schizophrenia. The identification of biological factors that underlie alterations in the aging process may permit identification of individuals at high risk for accelerated aging, and potentially open up consideration of new avenues for intervention.
The present review sought to evaluate evidence for accelerated biological aging in schizophrenia using systemic markers of biological aging. Though the recent literature has frequently used the term “accelerated biological aging,” no review has synthesized evidence for this claim at system level. Our goal was to conceptualize the issue and determine whether studies have appropriately addressed it. This hypothesis is most aptly addressed through longitudinal studies. However, in our search of the literature, we did not find any longitudinal investigations of systemic biomarkers in schizophrenia, although there are a few longitudinal studies of brain aging in schizophrenia.14,21 Nevertheless, we critically examined available cross-sectional studies to attempt to address 3 questions:
Are aging-related biomarkers abnormal in schizophrenia? We summarize the strength of the evidence for accelerated aging by examining how many reports found abnormal biomarker levels in individuals with schizophrenia compared to similarly aged HCs.
Which biomarkers are specifically involved in aging in schizophrenia? We summarize evidence suggesting a potentially altered pattern of age-related changes among patients with schizophrenia. Studies that explicitly compared the age-relationship between schizophrenia and HCs may provide the strongest available evidence for this hypothesis (supplementary table 1), although many did not perform such comparisons. As the next best option, studies that explored the age relationship individually within groups (without statistically comparing the relationships) were described.
What clinical characteristics in schizophrenia are risk vs protective factors for accelerated biological aging? We gathered information on which factors were associated with biomarkers in these studies and if they explained variance in biomarker levels over and above that due to chronological age.
Methods
We performed a search of PubMed, PsycINFO, and Embase with no year restrictions, for articles published before October 7, 2016 using the following search string: (schizophrenia OR schizoaffective OR psychosis[Title]) AND (aging OR ageing OR senescence OR “accelerated aging” OR “accelerated decline” OR “successful aging” OR premature OR elderly[Title]) AND (biomarker OR blood OR serum OR plasma OR urine OR cerebrospinal OR sweat OR inflammation OR immune OR oxidative OR telomere OR vascular OR metabolic OR insulin OR pulmonary) NOT (children[Title] OR childhood[Title] OR adolescence[Title] OR adolescent[Title] OR “first-episode”[Title] OR rodent [Title]). We examined the titles and abstracts of all citations and selected empirical reports based on our inclusion/exclusion criteria.
Inclusion/Exclusion Criteria
For inclusion in the review, studies had to: (1) include an age range of at least 30 years for mixed-age samples and 15 years for samples over age 60, (2) quantify levels of relevant biomarkers of systemic aging, and (3) evaluate the association between biomarker levels and chronological age in individuals with schizophrenia and comparison group(s). For the third criterion, studies must have reported results of age analyses such that a differential relationship between the schizophrenia and comparison group could be ascertained. Targeted outcomes for the present review included (but were not limited to) biomarkers of senescence, such as TL, pro-inflammatory indices, oxidative stress, and metabolic changes. As the focus of the paper was on biological indicators of systemic aging, we were interested in circulating biomarkers that would indicate overall physical/biological aging, are not dependent on a particular organ, could be relatively easily measured in blood, and are not influenced by motivation, interest, or learning/memory (thus, we excluded studies of neurocognitive or neuroimaging measures). The only exception made to this rule was for biomarkers measured in homogenized postmortem brain tissue, as these markers could have counterparts in and reflect changes across the rest of the body and are not restricted to the brain; these markers are currently measurable systemically or would be measurable in the near future. The rationale for our focus on systemic biomarkers was that they are accessible, practical to collect, particularly over repeated measurements to assess ongoing processes. Inclusion was restricted to studies published in English and of clinical populations with a diagnosis of schizophrenia or schizoaffective disorder. We excluded review papers and meta-analyses, reports with duplicate data, case reports, intervention trials, studies including children, adolescents, or first-episode patients, and studies using animal models.
Review Process
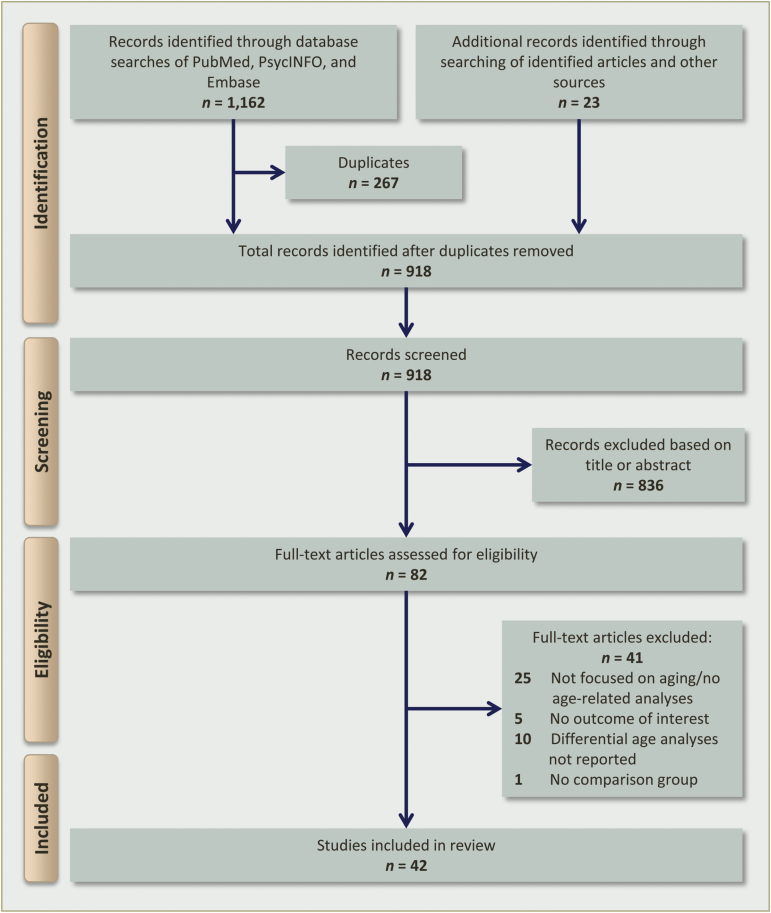
Our database search yielded 895 articles, once duplicates were removed. The titles and abstracts of all citations were screened and, of these, 59 met inclusion criteria for further review. Twenty-three additional articles were obtained from the references of identified papers. In total, 82 full-text articles were assessed for eligibility, and 41 met the above-mentioned criteria for review. One article22 reported on 2 experiments with different sample cohorts and biomarker samples, so this publication was counted twice as separate studies to make a total of 42 included studies. The PRISMA flow chart depicting information through different stages of the systematic review is shown in figure 1.
Fig. 1.
PRISMA flow diagram for selection of published articles for review.
Results
Characteristics of Reviewed Studies
Study characteristics and summary statistics for the 42 reviewed studies are presented in table 1. Summary information for each study is provided in supplementary table 2. Thirty-nine studies included individuals with schizophrenia and/or schizoaffective disorder, while 3 included patients with psychotic disorders, more generally.23–25 Seventy-one percent of reports were clinical investigations involving patient populations,22,23,25–52 whereas 29% examined systemic markers in postmortem brain tissue.22,53–63 Most studies investigated a mixed-age sample of adults, 2 included only older participants.55,58 All reviewed studies had at least one comparison group: 34 compared schizophrenia patients to a nonpsychiatric HC group; 4 involved at least one psychiatric comparison group in addition to an HC group;32,53,61,63 one included only psychiatric comparison groups40; one compared subtypes of patients with schizophrenia.34 The reviewed articles examined a variety of different biomarkers, including markers of inflammation (26%), TL (24%), indices of oxidative stress (17%), gene expression/regulation (10%), metabolic/vascular function (10%), receptor/synaptic function (10%), testosterone (2%), and brain-derived neurotropic factor (BDNF) (2%). In terms of analysis strategies, age was most often correlated with biomarker levels within each group separately (31%) or used as a covariate or predictor variable in a general linear model without specifying an interactive effect (31%). Only 24% of studies specifically explored an age × group interaction or statistically compared correlations/regression slopes between groups. Finally, 14% of studies compared subjects within or across age groups (eg, younger vs older subjects). Thirteen studies (31%) provided measures of effect sizes (or enough information to compute them).
Table 1.
Summary of Study Characteristics for Reports in Review
| Schizophrenia | Comparison | |||||
|---|---|---|---|---|---|---|
| Sample Characteristics | Mean | Median | Range | Mean | Median | Range |
| Number of Participants | 112.3 | 43.0 | 10–1457 | 282.5 | 43.5 | 9–8866 |
| Mean age (years) | 45.8 | 43.4 | 27–77 | 44.8 | 43.6 | 23–82 |
| Minimum age (years) | 27.1 | 21.5 | 14–67 | 26.7 | 23.0 | 11–64 |
| Maximum age (years) | 70.8 | 65.5 | 49–97 | 69.5 | 66.0 | 35–100 |
| Gender ratio (M/F) | 2.2 | 1.5 | 0.28–9.0 | 1.8 | 1.3 | 0.17–9.0 |
| Mean education (years) | 11.1 | 11.3 | 9–12 | 13.2 | 14.5 | 8–17 |
| Race (% Caucasian) | 56.8 | 56.3 | 0–100 | 58.6 | 61.8 | 0–100 |
| Mean age of onset (years) | 24.7 | 24.0 | 22–28 | — | — | — |
| Mean duration of illness (years) | 21.5 | 18.2 | 14–35 | — | — | — |
| Mean anti-psychotic dose (CPZE mg) | 538.7 | 512.0 | 222–759 | — | — | — |
| Biomarker categories (no. of studies) | 11 inflammation, 10 telomere length, 7 oxidative stress, 4 gene expression, 4 metabolic/vascular, 4 receptor/neurotransmitter systems, 1 testosterone, 1 BDNF | |||||
Note: BDNF, brain-derived neurotrophic factor; CPZE, chlorpromazine equivalent; F, females; M, males.
Below we summarize results for each set of biomarkers. Within each category, we first present data regarding group differences between schizophrenia and HCs (question 1). Next, we evaluate data examining differential age relationships between groups (question 2). Finally, we present associations of biomarkers with other factors in schizophrenia, such as clinical characteristics (question 3).
Inflammation.
Human aging is characterized by a chronic, low-grade inflammation, known as “inflammaging,” an important risk factor for both morbidity and mortality in older adults, which can be potentially prevented and even cured.64 A recent review65 concluded that the evidence on inflammation in schizophrenia is provocative.
Group Differences
Eleven studies measuring inflammatory biomarkers met our review criteria. Consistent with previous reviews and meta-analyses,66,67 73% of these reports observed elevated levels of pro-inflammatory cytokines and chemokines in schizophrenia, including interleukin (IL)-6,23,28,38,48 IL-1β,38,57 tumor necrosis factor alpha (TNFα),48 Eotaxin-1,35,45 Eotaxin-2,35 monocyte chemoattractant protein-1, macrophage inflammatory protein-1β, thymus- and activation-regulated chemokine, macrophage-derived chemokine,45 and lower levels of the anti-inflammatory cytokine IL-2.30 For TNF-α and IL-6, group differences were reduced and in some cases nonsignificant after controlling for potential confounds.48 Elevations were observed for other biomarkers related to inflammation, including markers of astrocyte and microglial activation (eg, S100B, glial fibrillary acid protein, CD11b), which can further induce cytokine production,37,57 as well as enzymes and transcription factors for the arachidonic acid and nuclear factor kappa B (NF-κB) signaling pathways.57 Of the 11 articles, 3 reported effect sizes ranging from small to medium in size, for elevated levels in schizophrenia.28,45,48 Other studies found no differences for C-reactive protein (CRP)40 or intercellular adhesion molecule-1 (ICAM-1)63; however, a more inclusive review and meta-analysis of all studies of CRP (without the specific study inclusion criteria that we used) has shown consistent elevations in CRP in schizophrenia.68
Age Relationships
Thirty-six percent of articles reported differential relationships between inflammation and age in schizophrenia and HCs. Three observed correlations between older age and increased IL-6,28 S100B,37 and scores on a chemokine index,45 which included MDC and Eotaxin-1, in schizophrenia but not in HCs. The strengths of correlation coefficients suggest medium effects; however, these investigations did not statistically compare the age-relationship between groups. Prostaglandin-related genes were different between older schizophrenia patients and age-comparable HCs, which were not observed between younger schizophrenia patients and their HCs, suggestive of differential aging trajectories between the groups.59 Only one study investigated an age × group interaction, and did not observe a group-specific age association for any chemokine.45 The remaining 64% of studies did not observe significant relationships between age and levels of cytokines (eg, IL-1β, IL-2, IL-6, TNFα),30,38,48,57 chemokines,35 CRP,40 ICAM-1,63 or brain mRNA levels of IL-1 receptor protein, markers of astrocyte and microglial activation, NF-κB transcription factor subunits, or arachidonic cascade enzymes.57
Association With Other Factors
Illness duration was reportedly associated with inflammation in 3 studies, suggesting that elevated levels may be related to disease progression. Longer illness duration was associated with higher IL-6 levels28 and chemokine index;45 the strengths of these relationships were small to moderate in size, respectively. Additionally, Eotaxin-1 levels were significantly higher in chronic patients compared to HCs.35 No relationships were observed between IL-2, TNFα, IL-6, IL-1β, S100B, or ICAM-1 and illness duration, antipsychotic medication use or dose, or cigarette smoking.28,30,37,38,48,63
Cytotoxicity.
Group Differences
Ten studies of TL met our inclusion criteria. Consistent with previous meta-analyses,17,19 findings were mixed. Five reports found that TL and telomerase activity were decreased in schizophrenia,29,34,36,42,49 3 observed no difference in TL between schizophrenia and HC groups,31,32,51 and one reported increased TL in schizophrenia.33 Wolkowitz et al47 found a group × gender interaction, such that HC women had longer TL than women with schizophrenia. Two articles reported effect sizes, which were small in magnitude,32,47 with one study finding longer TL in schizophrenia and the other reporting shorter TL in women with schizophrenia.
Age Relationships
None of the TL studies reported differential age relationships between schizophrenia and HC groups. Five reports found no relationship between age and TL29,31,32,51 or telomerase activity,36 while 4 observed negative correlations between age and TL in both schizophrenia and comparison groups.33,34,47,49 None of these studies statistically examined group differences in age-relationships. Two studies investigated an age × group interaction. Wolkowitz et al47 failed to observe a significant effect. Yu et al42 found that TL seemed to decrease with age in HCs but not in persons with chronic schizophrenia, with small effect sizes, despite also finding that poor responders to treatment exhibited shorter TL.
Association With Other Factors
Chronic patients with longer disease duration had significantly shorter TL than early-onset patients, with a large effect size.34 Greater number of psychotic episodes and hospital admissions were also related to shorter TL.34 Conversely, no associations were observed between TL and age of onset, disease course,33 or antipsychotic dose.29,34
Oxidative Stress.
Group Differences
Seven studies of oxidative stress markers met review criteria. In line with previous studies and reviews,20 88% of these studies observed abnormalities in levels of markers of oxidative stress in schizophrenia, including elevated plasma F2-isoprostanes,46 increased 8-hydroxy-2′-deoxyguanosine (8-OHdG) and Ki-67 protein,55 reduced glutathione,53 and decreased plasma albumin and bilirubin.41 Two studies reported on an index of total antioxidant status, measured as the net activity derived from various antioxidants in plasma, and both found a lower total antioxidant response43,52 in schizophrenia compared to HCs, as well as higher levels of total plasma peroxide levels.52 The only marker for which no group differences were found was thioredoxin.44 Effect sizes were reported in 2 investigations, and they were medium in size43,46; both studies indicated higher oxidative stress in schizophrenia.
Age Relationships
Two studies provided strong support for accelerated aging. Antioxidant protein bilirubin was significantly and more strongly related to age in HCs, suggesting that schizophrenia patients may lack a normal increase in antioxidants with age.41 Additionally, total antioxidant capacity was significantly lower in older patients with schizophrenia compared to young and middle-aged patients as well as older HCs.52 The remaining 5 studies did not find significant age relationships43,44,46,53,55 in either schizophrenia or comparison groups. Only one investigation tested age × group interactions and did not find any differential effects.46
Association With Other Factors
Four studies examined which disease-related factors may contribute to oxidative stress in schizophrenia. Total plasma antioxidant status was lower in individuals with longer duration of illness and antipsychotic medication use, with small-to-medium effect sizes.43 Otherwise, no other associations were observed between oxidative stress levels and age of onset, illness duration, antipsychotic medication dose or duration, smoking, or alcohol/drug abuse.41,43,44,46,53,55
Gene Expression and Regulation.
Group Differences
Four studies investigated markers of gene expression and regulation. Three reports investigated group differences in expression levels. Levels of hsa-miR-34a and hsa-miR449a microRNAs, previously identified to be potential biomarkers for schizophrenia, were higher in patients with schizophrenia compared to HCs in a human sample, with medium to large effect sizes; however, in a post-mortem sample, no differences were observed between the groups.22 Additionally, individuals with schizophrenia exhibited decreased expression of 5 neuronally expressed genes, HTR2C (serotonin 5-hydroxytryptamine receptor 2C), TOMM70A (mitochondrial function/import), RGS4 (regulator of G protein signaling 4), PPM1E (protein phosphatase, Mg2+/Mn2+ dependent, 1E), and GAD1 (GABAergic neurotransmission), compared to HCs; no difference in histone acetylation (Ac-H3K9K14) levels in these gene promoter regions was detected.61
Age Relationships
Fifty percent of gene expression and regulation studies found steeper age relationships in schizophrenia. In the clinical sample cohort, hsa-miR-34a significantly interacted with age such that expression seemed to increase among patients with schizophrenia compared to an apparent age-related decline in HCs.22 Additionally, in genome-wide expression profiles from the human frontal cortex, the relative expression levels of genes most significantly correlated with age were different between younger and older HCs but not in schizophrenia patients, suggesting that patients express a higher level of age-related genes throughout their lifespan.60 Additionally, 29%–34% of the genes that were correlated with age in healthy subjects were dysregulated in subjects with early-stage schizophrenia. On the other hand, neither hsa-miR-34a expression22 nor histone acetylation levels61 were associated with age in schizophrenia.
Association With Other Factors
Levels of hsa-miR-34a were higher in patients with longer than shorter duration of illness with large effect sizes, suggesting that this microRNA may increase with disease severity.22 In another study, microRNAs levels were measured at different clinical time-points (ie, during acute hospitalization and 2 months into remission); expression levels were not affected by hospitalization or significant improvement in clinical symptoms.22
Metabolic and Vascular Markers.
Recent reviews and meta-analyses have shown that people with schizophrenia are more likely to have metabolic abnormalities, which significantly influence physical morbidity and mortality,69,70 although it is less clear whether these dysregulations are due to aging (in schizophrenia) itself, or other factors related to the disease, including use of antipsychotic medications.71–73
Group Differences
Four articles examined the association between age and metabolic biomarkers in schizophrenia. Three studies observed elevated levels of cardiovascular risk markers in patients with schizophrenia, compared to HCs. These included blood glucose, low-density lipoprotein (LDL), total cholesterol, and triglyceride levels,25 glucose tolerance,23 C-peptide, and leptin concentrations.26 Only a single study reported effect sizes for higher levels in schizophrenia, which were medium to large in magnitude.23 Results of insulin concentration and resistance were mixed, with one study showing higher levels of insulin resistance in schizophrenia26 and another finding no difference.23 Levels of HbA1c, adiponectin, and desacyl ghrelin were not different from HCs.
Age Relationships
Three articles did not find age to be significantly related to glucose concentration or tolerance,23,26 insulin concentration and resistance,26 leptin,26 C-peptide,26 or desacyl ghrelin.39 Additionally, in a large survey study,25 correlations between vascular risk indicators (eg, total cholesterol, LDL, trigylcerides, and glucose) and age were significantly weaker in patients with psychosis than those in a national comparator sample.
Association With Other Factors
None of these investigations examined association between metabolic factors and disease-related factors in schizophrenia.
Receptor and Synaptic Function.
Group Differences
Four studies of receptor function met the review criteria. Two articles investigated group differences. Nicotinic α7 acetylcholine receptor expression,54 which via the cholinergic anti-inflammatory pathway may modulate cytokine production,74 and plasma levels of D-serine27 were reduced in schizophrenia, the latter with a medium effect size.
Age Relationships
Levels of mRNAs encoding for synaptic proteins in the left middle and superior temporal gyri were negatively related to age in schizophrenia; the magnitude of regression slopes was significantly different from HCs, in whom no age relationship was detected.58,62 Observed effect sizes were large, and findings provided strong evidence of faster-than-normal aging in schizophrenia. Neither D-serine27 nor any nicotinic subunit expression54 was correlated with age in schizophrenia.
Association With Other Factors
None of the receptor/synaptic biomarkers was related to disease variables, including duration of illness or length of time off antipsychotic medications prior to death.27,54,58,62
Other Biomarkers.
Two studies investigated the role of testosterone and BDNF. Stress and/or inflammation can alter the circulating levels of BDNF,75 and levels of BDNF decrease with impaired glucose metabolism,76 suggesting that it may be a pathogenetic factor involved in systemic age-related disorders, including diabetes and atherosclerosis.
Group Differences
Free androgen index (FAI), an indicator of androgen activity, was significantly lower in individuals with psychosis, with a medium effect size.77 Brain levels of BDNF did not differ between schizophrenia and HCs,56 although other reviews suggest that BDNF is reduced in schizophrenia.78
Age Relationships
Age was negatively correlated with FAI in patients with psychosis, with a medium-to-large effect size, but not in HCs. A trend for a steady, linear decrease in prefrontal gray matter BDNF from age 20 to 80 years was observed in HCs, consistent with findings in normal aging.79 Among the patient group, however, there was a linear decline in younger patients (20–50 years) followed by a leveling off in older patients (50–80 years); statistically, the slopes were similar in the younger cohort but different in the older cohort. The investigators concluded that the observed patterns might be due to a healthy survivor bias and speculated that this finding suggests an accelerated relationship with age and that BDNF is a protective biomarker.
Association With Other Factors
Neither report examined associations between testosterone or BDNF and disease-related factors in schizophrenia.
Discussion
“Accelerated” biological aging is a term commonly used to describe the aging process in schizophrenia. While several lines of evidence provide a basis for this hypothesis (eg, increased cardiovascular morbidity and mortality), these alone do not completely prove this postulate. Currently, existing research has inadequately tested this hypothesis; the major limitation is study design. Longitudinal studies are needed to characterize developmental trajectories across the lifespan and directly answer the question of accelerated aging. Unfortunately, the current literature on systemic biomarkers of aging contains only cross-sectional studies, which confound within- and between-subject sources of variation.80 Although the gold standard, longitudinal designs have drawbacks that have likely contributed to their dearth in the schizophrenia and aging literature, including large required sample sizes, costly time/resources necessary to follow participants, and participant attrition. Given the limitations of the existing literature, the term “anticipated” rather than “accelerated” aging may be appropriate. In the absence of longitudinal data to fully evaluate the accelerated aging hypothesis, we reviewed the available cross-sectional studies, with a critical eye and the caveat that they cannot entirely address the question at hand, in order to highlight trends and generate suggestions for future research.
With regard to our first aim, the cross-sectional data revealed strong evidence of abnormal physiological biomarkers in schizophrenia. A majority of studies (74%; n = 28 of 38) reported at least one comparison for which biomarker levels were abnormal in patients with schizophrenia compared to HCs. A total of 98 comparisons were performed across all investigations, and 66 of them were positive (67.3%). Effect sizes were reported in 13 studies, which indicated medium to large effects on average. This observation was seen across most biomarker types, including indices of inflammation, TL, oxidative stress, metabolic health, as well as gene expression, synaptic function, and testosterone, suggesting that schizophrenia is associated with aging-related biochemical abnormalities.
When we evaluated the relationship of biomarkers to age, only a minority of investigations (29%; n = 12 of 42) revealed a differential pattern of age-related decline in schizophrenia compared to HCs. Of the different biomarkers, the greatest proportion (50%) of studies demonstrating relationships with age in schizophrenia involved synaptic function and gene expression. These were followed in frequency by investigations of inflammation, oxidative stress, testosterone, and BDNF, respectively. Of note, the quality of the evidence for accelerated aging was strongest for studies focused on oxidative stress, gene expression, and synaptic function, as these studies more often statistically compared age trends between schizophrenia and HCs to suggest faster-than-normal decline in patients. There is growing evidence that oxidative stress plays an important role in the aging process,81,82 and age appears to be an important factor modifying antioxidant defense system in schizophrenia.41 Additionally, schizophrenia may anticipate the aging process as patients express a higher level of age-related genes throughout their lifespan and share common molecular signatures with normal aging subjects at earlier ages,60 and normal cortical development may be altered or delayed, with irregularities in programmed synaptic elimination in schizophrenia.58,62
Studies of TL and metabolic indices were least likely to show results consistent with a faster rate of aging in schizophrenia. None of these investigations revealed differential age-related decline in schizophrenia; in fact, studies in these categories showed the opposite finding—a decelerated trend in TL with age in schizophrenia42 and a stronger relationship between metabolic risk factors and age in HCs.25 Results among publications investigating markers of inflammation and oxidative stress were mixed, with 36% and 29% of reports, respectively, observing relationships between biological markers and age in schizophrenia.
Just as the observation of a cross-sectional correlation between biomarker level and age does not confirm the presence of accelerated aging in schizophrenia, its absence does not rule out the possibility. Potential reasons for null correlations are: (1) cohort effects (eg, younger patients may be more ill, less stable on medications), (2) sampling effects such as the “healthy survivor” bias,83 (3) nonlinear aging trajectories, especially after age 60, (4) narrow age ranges studied, and (5) slower progression compared to other classic aging disorders (eg, Alzheimer’s disease). Moreover, the lack of observed age relationships raises other issues, including (6) whether the lack of correlation disputes accelerated aging per se or suggests the particular biomarker’s nonrelevance to aging in schizophrenia, (7) whether the relationship to duration of illness is more important than to age, and (8) the possibility that subtypes of patients are at higher risk for accelerated aging.
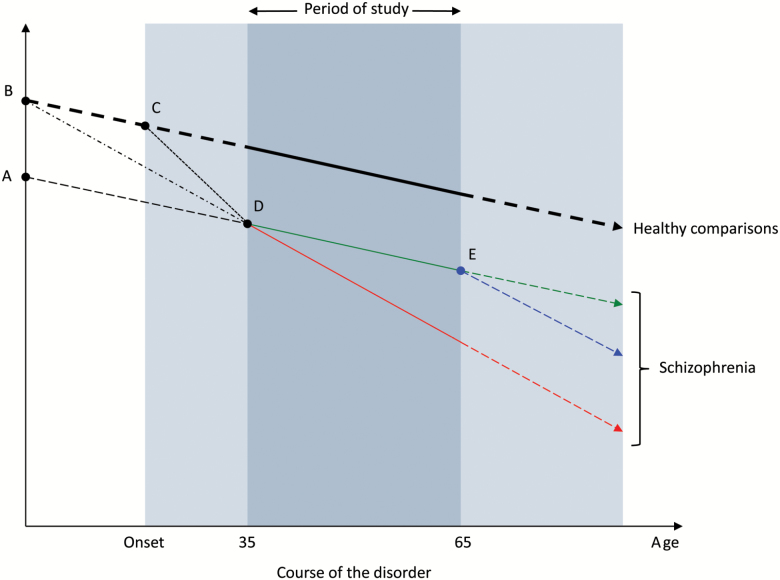
In considering the results of this review, focused on biomarkers measured during adulthood, it is important to realize that there are several possible models of biological aging in schizophrenia (figure 2), distinguished by when and whether there is a differential rate of age-related change during different age ranges. If no differential age-related change is noted during a particular part of the lifespan, this does not mean that the rate of change has been the same or will continue to parallel the trajectory of healthy individuals. Similarly, if aging is found to be accelerated during a given period that does not imply that it was always so or will continue to accelerate. An important question, not addressed by the studies in the current review, is: does the accelerated aging process begin before or after the onset of clinical symptoms of the disorder? For example, ongoing research on prodromal samples may be able to address the timing of alterations in the aging process.84
Fig. 2.
Models of altered aging in schizophrenia. The entire shaded area represents the time period during which an individual has the disorder. The darker shaded area represents the period of measurement in a given study (for this example, a study includes individuals between the ages of 35 to 65 years). Solid lines indicate trajectories that are observed within the measurement period and dashed lines represent unobserved trajectories that occur beyond (either before or after) the period of study. The heavier weighted line indicates the trajectory of healthy, nonpsychiatric individuals, while lighter weighted lines represent possible trajectories in schizophrenia. Points A through E signify inflection points during the life course, at which time, changes in biomarker trajectories might occur.
The current biomarker literature suggests that aging is altered in schizophrenia, and the mechanisms are likely to be multifactorial. The observed aging patterns may be a result of the pathophysiology of schizophrenia or, given the oft-observed correlations with duration of illness, due to the wear and tear on the body from factors associated with the illness, such as medication effects, the stress of psychotic episodes, stress of lower functional status, weak social support, poor healthcare, childhood abuse/trauma, lower socioeconomic status, and lifestyle factors such as unhealthy diet, sedentary behavior, and heavy smoking. From the available literature, aging processes may begin early in the lifespan through abnormalities in genetic factors and altered synaptic function, which may then be exacerbated by inflammation and oxidative stress associated with illness-related factors.
Further exploration of aging patterns and their mechanisms is critical as they have important implications for treatment. Better understanding of inflection points when biomarker levels begin to decline (figure 2) may impact the timing and/or goal of intervention programs. For instance, if decline occurs around the time of disease onset (Point C), prevention efforts might be implemented during the prodromal period. Conversely, if decline begins from birth (Point B), interventions might be aimed at slowing the rate of decline. If individuals with schizophrenia are born with abnormalities in aging-related biomarkers (Point A), these biological factors would be important trait markers that can help identify at-risk individuals who may benefit most from preventative strategies to prevent further decline.
Additional limitations of the present review should be acknowledged. Studies in this area are weakened by a failure to match comparison groups on relevant variables that include, but are not limited to, gender, ethnicity, smoking, substance use, diet, body mass index, socioeconomic status, and stress. For instance, differences in gender ratios across groups may impact findings across investigations. Few studies matched groups on all of these variables (or at least a subset beyond age and gender)23,32,35,44,77 or considered antipsychotic exposure.26,28,29,34,41,43,44,46,48,54,55 Moreover, none of the reports mentioned whether patients with late-onset schizophrenia were included in their samples so it is unclear if a different age relationship might be seen in such patients. Despite our best efforts and thorough literature search, it is possible that we may have failed to include some relevant papers. Also, as with any review, publication biases might have influenced a predominance of positive results. Relatedly, because this review focused on a subset of studies that specifically examined the relationship of biomarkers to age, studies that investigated differences in schizophrenia on these measures but did not report any age-related analyses were not included.85 Despite these limitations, this review offers a novel perspective on the existing literature and debate regarding aging in schizophrenia.
The clinical implications of accelerated biological aging are substantial. People with schizophrenia develop medical comorbidity at earlier ages and die younger. A focus on understanding the mechanisms of these changes, seeking to remediate or prevent decline, is needed. Biomarkers provide insights into candidate mechanisms that may underlie deleterious aging processes. Future studies to test the hypothesis of accelerated biological aging should include the following:
Longitudinal study designs with a wide range of baseline ages: Structured multi-cohort longitudinal designs are particularly well-suited to address this question.80 With cohort age spans that overlap, age cohort effects can be statistically tested against developmental effects.86,87
Longer studies: Schizophrenia is unlike other disorders of aging (eg, Alzheimer’s disease) in which individuals decline rapidly after diagnosis. Trajectories are likely to be considerably slower in schizophrenia and may not be demonstrable with truncated longitudinal follow-up.
Larger sample sizes: Given considerable inter-individual variations in biomarker levels, large samples are needed to detect inter-individual variability.
Frequent assessments to detect inflection points.
Exploration of schizophrenia-specific covariates, such as age of onset, duration of illness, antipsychotic dose, lifetime medication burden, etc.
Emphasis on systemic markers of neuronally-relevant gene expression, synaptic function, and antioxidants, as these have the strongest evidence for differential age relationships in cross-sectional studies. An area of particular potential interest and importance is epigenetics, which change with increasing age and contribute to age-related disease.88 DNA methylation has been shown to be a systemic biomarker of biological age, and differences in methylation patterns between an individual’s estimated biological age and chronological age have been shown to be clinically meaningful in various populations.88,89 Only one study has addressed the accelerated biological aging hypothesis in schizophrenia using this approach.90 However, additional studies are needed.
In conclusion, the jury is still out on the hypothesis of accelerated biological aging in schizophrenia. There is some suggestion for accelerated aging but no clear demonstration. However, current findings in the areas of oxidative stress, gene expression, and synaptic function are promising and pave the way for future research. Confirmation of the hypothesis of accelerated biological aging and identification of relevant biomarkers could help identify high-risk patients and contribute to the development of potentially life-prolonging interventions.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by National Institute of Mental Health (grant numbers 5R01 MH094151-04 and 5T32 MH019934-21 to D.V.J.), the Desert-Pacific Mental Illness Research, Education, and Clinical Center (T.T.N. and L.T.E.), the Department of Veterans Affairs Office of Academic Affiliations (T.T.N.), and UC San Diego Stein Institute for Research on Aging.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraepelin E. Dementia Praecox and Paraphrenia. Huntington, NY: Krieger Publishing Company; 1971. [Google Scholar]
- 2. Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging?Schizophr Bull. 2008;34:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. [DOI] [PubMed] [Google Scholar]
- 4. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell AJ, Malone D. Physical health and schizophrenia. Curr Opin Psychiatry. 2006;19:432–437. [DOI] [PubMed] [Google Scholar]
- 6. D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 7. Goff DC, Sullivan LM, McEvoy JP et al. . A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. [DOI] [PubMed] [Google Scholar]
- 8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?Arch Gen Psychiatry. 2007;64:1123–1131. [DOI] [PubMed] [Google Scholar]
- 9. Tsuang MT, Woolson RF. Excess mortality in schizophrenia and affective disorders. Do suicides and accidental deaths solely account for this excess?Arch Gen Psychiatry. 1978;35:1181–1185. [DOI] [PubMed] [Google Scholar]
- 10. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–508. [DOI] [PubMed] [Google Scholar]
- 11. Casey DE, Hansen TE, Meyer J, Nasrallah H. Excessive mortality and morbidity associated with schizophrenia. In: Meyer JM, Nasrallah HA, eds. Medical illness and schizophrenia. Washington, DC: American Psychiatric Press, Inc; 2009:17–35. [Google Scholar]
- 12. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am J Psychiatry. 2016;173:607–616. [DOI] [PubMed] [Google Scholar]
- 15. Chiapponi C, Piras F, Fagioli S, Piras F, Caltagirone C, Spalletta G. Age-related brain trajectories in schizophrenia: a systematic review of structural MRI studies. Psychiatry Res. 2013;214:83–93. [DOI] [PubMed] [Google Scholar]
- 16. Koutsouleris N, Davatzikos C, Borgwardt S et al. . Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. Leukocyte telomere length in patients with schizophrenia: a meta-analysis. Schizophr Res. 2015;165:195–200. [DOI] [PubMed] [Google Scholar]
- 18. Shivakumar V, Kalmady SV, Venkatasubramanian G, Ravi V, Gangadhar BN. Do schizophrenia patients age early?Asian J Psychiatr. 2014;10:3–9. [DOI] [PubMed] [Google Scholar]
- 19. Lin PY. Shortened leukocyte telomere length in patients with schizophrenia is related to disease status. Schizophr Res. 2015;168:597–598. [DOI] [PubMed] [Google Scholar]
- 20. Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. [DOI] [PubMed] [Google Scholar]
- 22. Lai CY, Lee SY, Scarr E et al. . Aberrant expression of microRNAs as biomarker for schizophrenia: from acute state to partial remission, and from peripheral blood to cortical tissue. Transl Psychiatry. 2016;6:e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandez-Egea E, Bernardo M, Donner T et al. . Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009;194:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Egea E, Bernardo M, Heaphy CM et al. . Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foley DL, Mackinnon A, Morgan VA et al. . Cardiovascular risk factor associations in adults with psychosis and adults in a national comparator sample. Aust N Z J Psychiatry. 2015;49:714–723. [DOI] [PubMed] [Google Scholar]
- 26. Arranz B, Rosel P, Ramírez N et al. . Insulin resistance and increased leptin concentrations in noncompliant schizophrenia patients but not in antipsychotic-naive first-episode schizophrenia patients. J Clin Psychiatry. 2004;65:1335–1342. [DOI] [PubMed] [Google Scholar]
- 27. Calcia MA, Madeira C, Alheira FV et al. . Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr Res. 2012;142:83–87. [DOI] [PubMed] [Google Scholar]
- 28. Ganguli R, Yang Z, Shurin G et al. . Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res. 1994;51:1–10. [DOI] [PubMed] [Google Scholar]
- 29. Kao HT, Cawthon RM, Delisi LE et al. . Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. [DOI] [PubMed] [Google Scholar]
- 30. Mahendran R, Mahendran R, Chan YH. Interleukin-2 levels in chronic schizophrenia patients. Ann Acad Med Singapore. 2004;33:320–323. [PubMed] [Google Scholar]
- 31. Malaspina D, Dracxler R, Walsh-Messinger J et al. . Telomere length, family history, and paternal age in schizophrenia. Mol Genet Genomic Med. 2014;2:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mansour H, Chowdari K, Fathi W et al. . Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia?Psychiatry Res. 2011;188:129–132. [DOI] [PubMed] [Google Scholar]
- 33. Nieratschker V, Lahtinen J, Meier S et al. . Longer telomere length in patients with schizophrenia. Schizophr Res. 2013;149:116–120. [DOI] [PubMed] [Google Scholar]
- 34. Pawelczyk T, Szymanska B, Grancow-Grabka M, Kotlicka-Antczak M, Pawelczyk A. Telomere length in blood cells is related to the chronicity, severity, and recurrence rate of schizophrenia. Neuropsychiatr Dis Treat. 2015;11:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pedrini M, Massuda R, de Lucena D et al. . Differences in eotaxin serum levels patients with recent onset and in chronic stable schizophrenia: a clue for understanding accelerating aging profile. Schizophr Res. 2014;152:528–529. [DOI] [PubMed] [Google Scholar]
- 36. Porton B, Delisi LE, Bertisch HC et al. . Telomerase levels in schizophrenia: a preliminary study. Schizophr Res. 2008;106:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitt A, Bertsch T, Henning U et al. . Increased serum S100B in elderly, chronic schizophrenic patients: negative correlation with deficit symptoms. Schizophr Res. 2005;80:305–313. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt A, Bertsch T, Tost H et al. . Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr Dis Treat. 2005;1:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wysokiński A, Kowalski ML, Kłoszewska I. Serum levels of desacyl ghrelin in patients with schizophrenia on clozapine monotherapy. Psychiatry Clin Neurosci. 2014;68:833–840. [DOI] [PubMed] [Google Scholar]
- 40. Wysokiński A, Margulska A, Strzelecki D, Kłoszewska I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord J Psychiatry. 2015;69:346–353. [DOI] [PubMed] [Google Scholar]
- 41. Yao JK, Reddy R, van Kammen DP. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 2000;97:137–151. [DOI] [PubMed] [Google Scholar]
- 42. Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang XY, Chen DC, Xiu MH et al. . Plasma total antioxidant status and cognitive impairments in schizophrenia. Schizophr Res. 2012;139:66–72. [DOI] [PubMed] [Google Scholar]
- 44. Zhang XY, Chen DC, Xiu MH et al. . Thioredoxin, a novel oxidative stress marker and cognitive performance in chronic and medicated schizophrenia versus healthy controls. Schizophr Res. 2013;143:301–306. [DOI] [PubMed] [Google Scholar]
- 45. Hong S, Lee EE, Martin AS et al. . Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophr Res. 2016;181:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee EE, Eyler LT, Wolkowitz OM et al. . Elevated plasma F2-isoprostane levels in schizophrenia. Schizophr Res. 2016;176:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolkowitz OM, Jeste DV, Martin AS et al. . Leukocyte telomere length: effects of schizophrenia, age, and gender. J Psychiatr Res. 2017;85:42–48. [DOI] [PubMed] [Google Scholar]
- 48. Lee EE, Hong S, Martin AS, Eyler LT, Jeste DV. Inflammation in Schizophrenia: cytokine levels and their relationships to demographic and clinical variables. Am J Geriatr Psychiatry. 2017;25:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Czepielewski LS, Massuda R, Panizzutti B et al. . Telomere length in subjects with schizophrenia, their unaffected siblings and healthy controls: evidence of accelerated aging. Schizophr Res. 2016;174:39–42. [DOI] [PubMed] [Google Scholar]
- 50. Liang Y, Huang J, Tian JB et al. . Factors associated with decreased bone mineral density in postmenopausal women with schizophrenia. Clin Interv Aging. 2016;11:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malaspina D, Walsh-Messinger J, Antonius D et al. . Parental age effects on odor sensitivity in healthy subjects and schizophrenia patients. Am J Med Genet B Neuropsychiatr Genet. 2016;171:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uma Devi P, Devipriya D, Chinnaswamy P. Age and gender related changes in total antioxidant response and oxidative stress in patients with schizophrenia. J Clin Diagn Res. 2008;2:627–633. [Google Scholar]
- 53. Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. [DOI] [PubMed] [Google Scholar]
- 54. Martin-Ruiz CM, Haroutunian VH, Long P et al. . Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–1233. [DOI] [PubMed] [Google Scholar]
- 55. Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12:167–175. [PubMed] [Google Scholar]
- 56. Rao J, Chiappelli J, Kochunov P, Regenold WT, Rapoport SI, Hong LE. Is schizophrenia a neurodegenerative disease? Evidence from age-related decline of brain-derived neurotrophic factor in the brains of schizophrenia patients and matched nonpsychiatric controls. Neurodegener Dis. 2015;15:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res. 2013;147:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sokolov BP, Tcherepanov AA, Haroutunian V, Davis KL. Levels of mRNAs encoding synaptic vesicle and synaptic plasma membrane proteins in the temporal cortex of elderly schizophrenic patients. Biol Psychiatry. 2000;48:184–196. [DOI] [PubMed] [Google Scholar]
- 59. Tang B, Capitao C, Dean B, Thomas EA. Differential age- and disease-related effects on the expression of genes related to the arachidonic acid signaling pathway in schizophrenia. Psychiatry Res. 2012;196:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang B, Chang WL, Lanigan CM, Dean B, Sutcliffe JG, Thomas EA. Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell. 2009;8:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang B, Dean B, Thomas EA. Disease- and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl Psychiatry. 2011;1:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tcherepanov AA, Sokolov BP. Age-related abnormalities in expression of mRNAs encoding synapsin 1A, synapsin 1B, and synaptophysin in the temporal cortex of schizophrenics. J Neurosci Res. 1997;49:639–644. [DOI] [PubMed] [Google Scholar]
- 63. Thomas AJ, Davis S, Ferrier IN, Kalaria RN, O’Brien JT. Elevation of cell adhesion molecule immunoreactivity in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry. 2004;55:652–655. [DOI] [PubMed] [Google Scholar]
- 64. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 65. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. [DOI] [PubMed] [Google Scholar]
- 67. Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93–115. [DOI] [PubMed] [Google Scholar]
- 68. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7:223–230. [DOI] [PubMed] [Google Scholar]
- 69. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand. 2015;132:144–157. [DOI] [PubMed] [Google Scholar]
- 71. Potvin S, Zhornitsky S, Stip E. Antipsychotic-induced changes in blood levels of leptin in schizophrenia: a meta-analysis. Can J Psychiatry. 2015;60:S26–S34. [PMC free article] [PubMed] [Google Scholar]
- 72. Bartoli F, Lax A, Crocamo C, Clerici M, Carrà G. Plasma adiponectin levels in schizophrenia and role of second-generation antipsychotics: a meta-analysis. Psychoneuroendocrinology. 2015;56:179–189. [DOI] [PubMed] [Google Scholar]
- 73. Stubbs B, Wang AK, Vancampfort D, Miller BJ. Are leptin levels increased among people with schizophrenia versus controls? A systematic review and comparative meta-analysis. Psychoneuroendocrinology. 2016;63:144–154. [DOI] [PubMed] [Google Scholar]
- 74. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aloe L, Tirassa P, Bracci-Laudiero L. Nerve growth factor in neurological and non-neurological diseases: basic findings and emerging pharmacological prospectives. Curr Pharm Des. 2001;7:113–123. [DOI] [PubMed] [Google Scholar]
- 76. Krabbe KS, Nielsen AR, Krogh-Madsen R et al. . Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. [DOI] [PubMed] [Google Scholar]
- 77. Fernandez-Egea E, García-Rizo C, Miller B et al. . Testosterone in newly diagnosed, antipsychotic-naive men with nonaffective psychosis: a test of the accelerated aging hypothesis. Psychosom Med. 2011;73:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. [DOI] [PubMed] [Google Scholar]
- 79. Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. [DOI] [PubMed] [Google Scholar]
- 80. Thompson WK, Hallmayer J, O’Hara R; Alzheimer’s Disease Neuroimaging Initiative Design considerations for characterizing psychiatric trajectories across the lifespan: application to effects of APOE-ε4 on cerebral cortical thickness in Alzheimer’s disease. Am J Psychiatry. 2011;168:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. [DOI] [PubMed] [Google Scholar]
- 82. Harman D. Free-radical theory of aging. Increasing the functional life span. Ann N Y Acad Sci. 1994;717:1–15. [DOI] [PubMed] [Google Scholar]
- 83. Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we?Am J Psychiatry. 2000;157:163–171. [DOI] [PubMed] [Google Scholar]
- 84. Addington J, Cadenhead KS, Cornblatt BA et al. . North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garcia-Rizo C, Fernandez-Egea E, Oliveira C et al. . Prolactin concentrations in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis. Schizophr Res. 2012;134:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. BELL RQ. An experimental test of the accelerated longitudinal approach. Child Dev. 1954;25:281–286. [PubMed] [Google Scholar]
- 87. BELL RQ. Convergence: an accelerated longitudinal approach. Child Dev. 1953;24:145–152. [PubMed] [Google Scholar]
- 88. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bocklandt S, Lin W, Sehl ME et al. . Epigenetic predictor of age. PLoS One. 2011;6:e14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA. DNA methylation evidence against the accelerated aging hypothesis of schizophrenia. NPJ Schizophr. 2017;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.