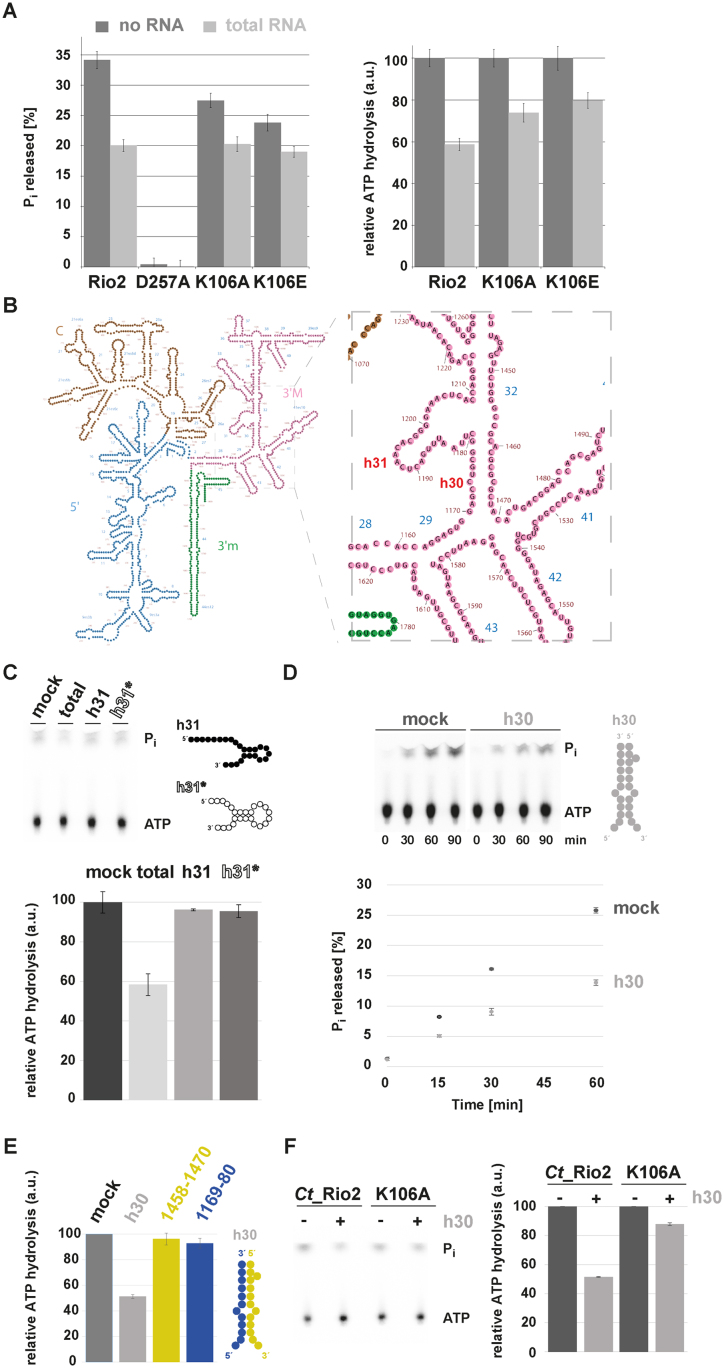
Figure 7.
P-loop lysine and h30 participate in the RNA-dependent regulation of Rio2 ATPase activity. (A) RNA-dependent regulation of Ct_Rio2 ATPase activity. Single-turnover experiments using the indicated recombinant proteins (≈0.5 μM protein) were performed in presence of 2 μg/μl of yeast total RNA. Percentage of released Pi (left panel) and the deduced relative ATPase activity (right panel) are shown. (B) Yeast 18S rRNA 2D structure. 2D structure prediction were obtained from http://apollo.chemistry.gatech.edu/RibosomeGallery (72). The 5′ domain (blue), Central domain (brown), 3′Major domain (magenta) and 3′minor domain (green) are depicted. A close-up of the h30 and h31 region is depicted in the right panel. (C) ATPase activity analysis in the presence of an h31 rRNA-mimic. Relative ATPase activity was monitored during 30 min by single-turnover experiment in presence of 2 μg/μl total yeast RNA or 10 μM of h31 derivatives (≈0.5 μM protein). Helix 31* is a stabilized version of h31 to which a series of A–U base pairing to stabilize the stem of this helix was added (see also Supplementary Figure S3). 2D RNA structure predictions are schematically represented (see Supplementary Figure S3 for further information). (D) ATPase activity analysis in the presence of an h30 rRNA-mimic. Time-dependent Pi released (in %) as obtained with Ct_Rio2 (≈0.5 μM) incubated in presence of an excess (10 μM) of an annealed h30-mimic is shown. 2D RNA structure predictions are schematically represented (see Supplementary Figure S3 for further information). (E) Double strand RNA integrity of h30 is required for RNA-dependent regulation of Ct_Rio2 ATPase activity. Relative ATPase activity was monitored during 30 min by single-turnover experiment as obtained with Ct_Rio2 (≈0.5 μM) incubated in presence of an excess (10 μM) of an annealed h30-mimic and (20 μM) of each individual single strand RNA region forming h30 (18S rRNA 1169–80 and 1458–70 Sc numbering, depicted in blue and yellow respectively). All quantitation and standard deviation were derived from 4 independent experiments. (F) Ct_Rio2 P-loop lysine integrity is required for efficient h30-dependent Rio2 ATPase activity inhibition. Relative ATPase activity was monitored during 30 min by single-turnover experiment as obtained with Ct_Rio2 and Ct_Rio2 K105A (≈0.5 μM) incubated in presence of an excess (10 μM) of annealed h30-mimic is shown.