Figure 8.
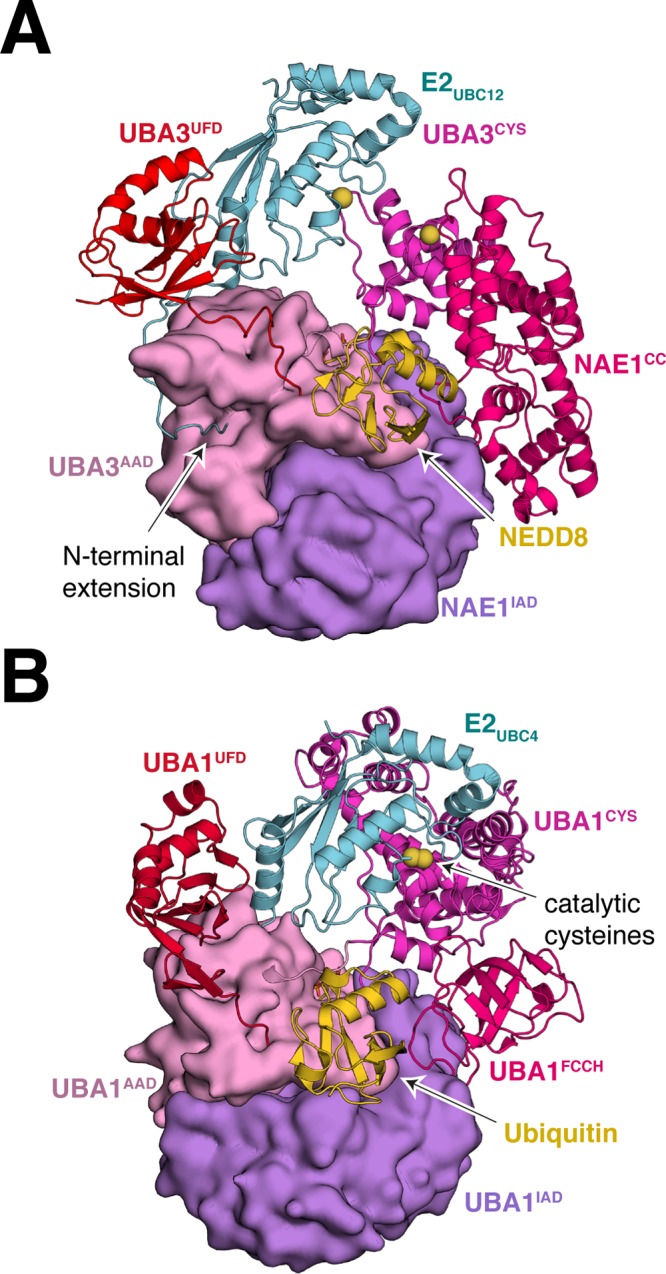
E1–E2 interaction for canonical E1 proteins. (A) Structure of human E1NAE1/UBA3/E2UBC12/NEDD8/ATP (PDB 2NVU) showing the bipartite binding of the E2UBC12 to E1. In this structure, the catalytic cysteine residues of E1 and E2 are separated by ∼20 Å. (B) Structure of S. pombe E1UBA1/E2UBC4/ubiquitin/ATP (PDB 4II2) showing juxtaposition of E1 and E2 active sites. Adenylation domains are shown in Gaussian surface representation. Other domains are in cartoon representation. The sulfur atoms of the active site cysteine residues of E1 and E2 are in sphere representation colored yellow.
