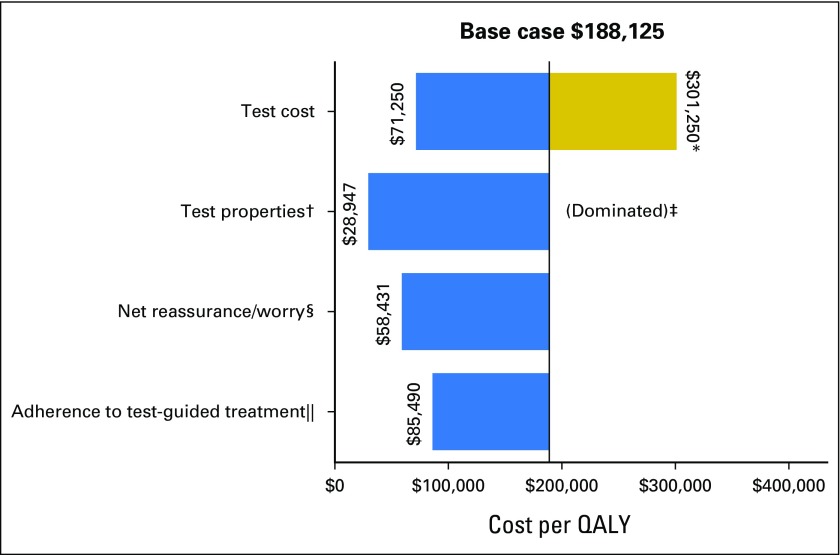
Fig 2.
Impact of varying single parameters on the societal cost-effectiveness ratios for Oncotype DX testing in community practice versus usual care without testing among patients with stage I or II, node-negative, estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer. This diagram illustrates the changes in the incremental cost-effectiveness ratio (ICER) for the costs per quality-adjusted life-year (QALY) under various parameter values and alternative assumptions. The solid vertical line represents the base-case ICER result comparing community practice with Oncotype DX testing versus usual care without Oncotype DX. The horizontal bars indicate the change from the base ICER when the one individual parameter is varied. If the bar goes to the right of the base case, it indicates that the alternative value or assumption costs more per QALY than the base case, where bars that go to the left indicate that the alternative value or assumption costs less per QALY than the base case. (*) Test costs were varied from the base case of $3,416 to $2,657 and $4,175. The large difference in cost per QALY when test costs were varied is a result of these effects being magnified by the small incremental QALYs between usual care and community care. (†) The accuracy of the test represents the probability of a test score, conditional on actual distant recurrence. The best test accuracy reflects a greater proportion of women who actually experience recurrence having high-risk scores and a smaller proportion having low-risk scores, and among those who do not experience recurrence, fewer have high-risk scores and more have low-risk scores than in the base case on the basis of observed performance in the original validation study. (‡) The worst testing accuracy was dominated. That is, it resulted in community practice being more costly and producing fewer QALYs than usual care without testing. (§) Net reassurance/worry is based on gaining 0.5 QALY or losing 0.5 QALY over the first 2 years after diagnosis with low- and high-risk recurrence scores, respectively. (‖) One hundred percent adherence to test-guided treatment assumes 100% chemotherapy use among patients with high-risk score on gene expression profile testing, 50% chemotherapy use for intermediate risk, and 0% chemotherapy use for low risk.