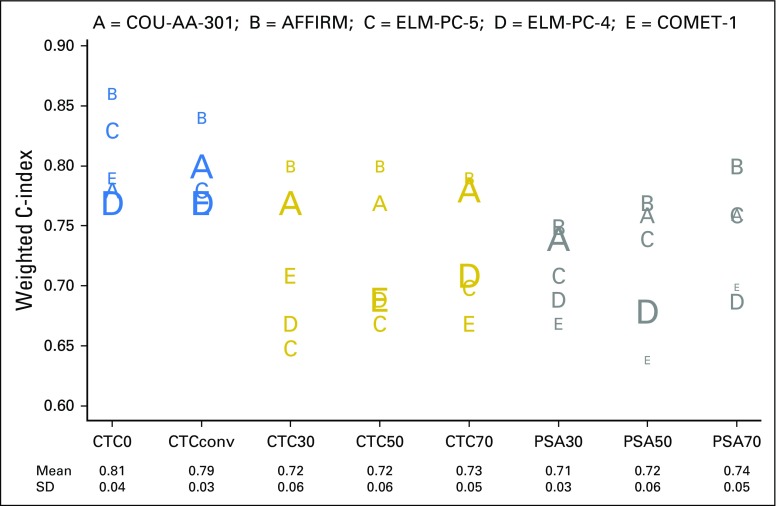
Fig 2.
Discriminatory power of post-therapy circulating tumor cell (CTC) and prostate-specific antigen (PSA) response measures for survival in metastatic castration-resistant prostate cancer registration trials. CTC0, CTC count ≥ 1 at baseline and 0 at week 13; CTCconv, CTC conversion (CTC count ≥ 5 at baseline and ≤ 4 at week 13); CTC30, CTC count ≥ 5 at baseline and a 30% decline from baseline to week 13; CTC50, CTC count ≥ 5 at baseline and a 50% decline from baseline to week 13; CTC70, CTC count ≥ 5 at baseline and a 70% decline from baseline to week 13; PSA30, PSA level ≥ 5 ng/mL at baseline and a 30% decline from baseline to week 13; PSA50, PSA level ≥ 5 ng/mL at baseline and a 50% decline from baseline to week 13; PSA70, PSA level ≥ 5 ng/mL at baseline and a 70% decline from baseline to week 13. SD, standard deviation. ClinicalTrials.gov identifiers for the trials are: COU-AA-301, NCT00638690; AFFIRM, NCT00974311; ELM-PC-5, NCT01193257; ELM-PC-4, NCT01193244; COMET-1, NCT01605227.