Figure 1.
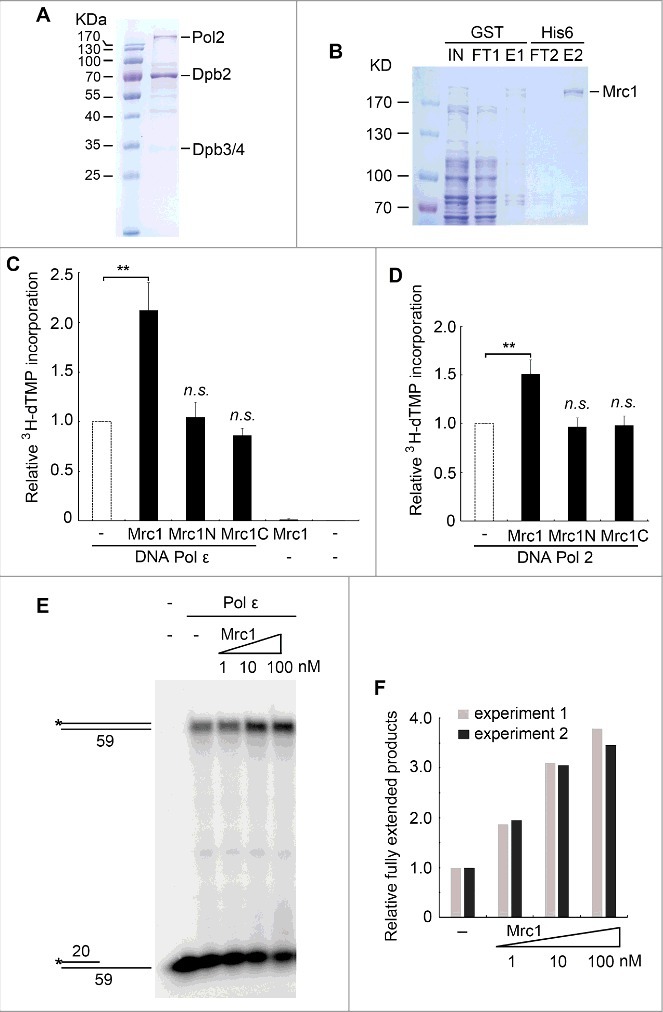
Mrc1 stimulates the DNA polymerase activity of Pol ε in vitro. (A) Purification of the Pol ε complex containing the catalytic Pol2, and accessory Dpb2, Dpb3 and Dpb4 subunits from yeast. See Experimental Procedures for details. (B) Purification of recombinant GST-Mrc1-His6 through two sequential affinity chromatography as described in Experimental Procedures. (C) The 3H-dTMP incorporation by Pol ε is significantly increased in the presence of full-length Mrc1 protein. DNA Pol ε (1 nM) was incubated with poly(dA)-oligo(dT) in a total volume of 60 μL at 37°C for 30min. Mrc1 or its fragment Mrc1N (1-567)/Mrc1C(568-1096) (1 nM) was added to the reactions as indicated. The 3H-dTMP incorporation was measured by scintillation counting. Averages from at least three independent experiments ± SD were shown. Statistical significance was measured using Student's t-test. **P < 0.01; n.s., not significant. (D) The DNA polymerase activity of Pol2 can be stimulated by Mrc1 protein as well. The experiments were performed as above except that Pol2 was used instead of Pol ε. Mean ± SD are shown from three independent experiments. Statistical significance was measured using Student's t-test. **P < 0.01; n.s., not significant. (E) The stimulation effect of Mrc1 was validated by primer extension assays. The increasing amount of Mrc1 protein (1-100 nM) was added into the primer extension reactions containing 1 nM Pol ε at 37°C. The reactions were quenched and subjected to 8% PAGE containing 7M urea followed by autoradiography. (F) Quantification of two biological repeats of (E) using Bio-Rad Quantity One.
