Figure 5.
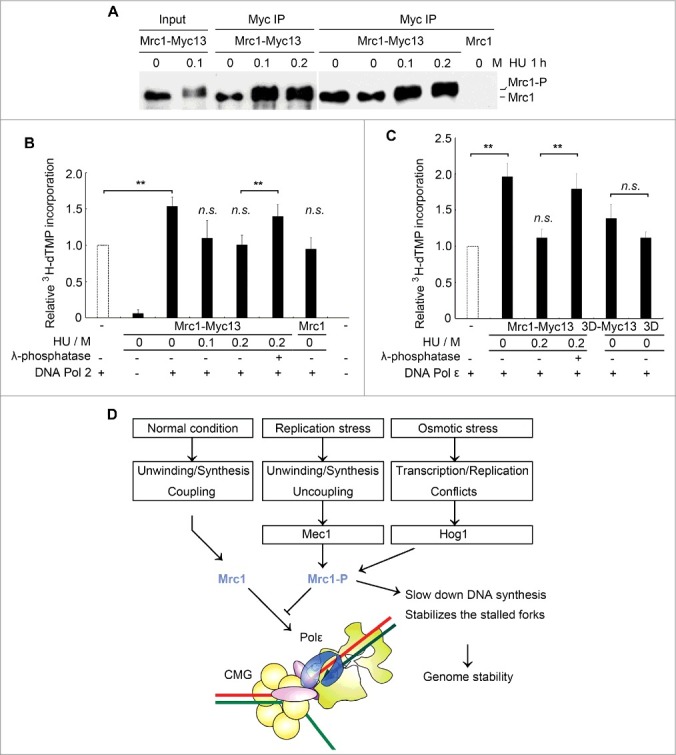
Mrc1 phosphorylation abolishes its stimulation effect on Pol2. (A) Endogenously myc13-tagged Mrc1 protein was precipitated by anti-myc from cells with or without HU treatment for 1 h. Mrc1 protein migrated slowly when it was phosphorylated after HU treatment. (B) Phosphorylated Mrc1 loses the stimulation effect on DNA Pol2. The DNA polymerase activity was measured as described in 1C. Precipitates from an untagged MRC1 strain were used as controls. Mean ± SD are shown from three independent experiments. Statistical significance was measured using Student's t-test. **P < 0.01; n.s., not significant. (C) A brief summary of the roles of Mrc1 under normal or stressed conditions based on previous findings and this study. Under normal conditions, Mrc1 connects Pol ε with CMG holo-helicase. These physical interactions can stimulate the DNA synthesis activity of Pol ε and thus ensure the coupling between duplex unwinding by CMG helicase and DNA synthesis by Pol ε. When cells experience replication or osmotic stresses, Mrc1 becomes phosphorylated by Mec1 or Hog1. This abrogates the DNA synthesis stimulation, which may contribute to fork pausing and stabilization.
