Figure 6.
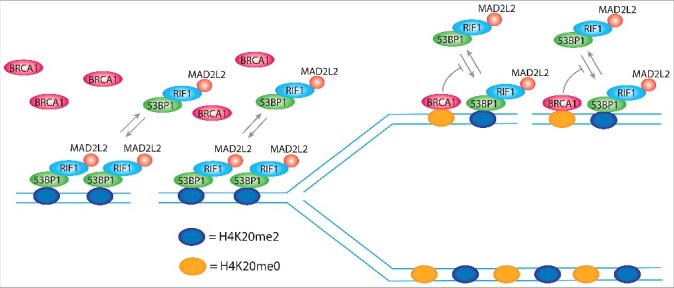
H4K20me2 levels control DNA repair pathway choice. 53BP1 forms foci at DSBs by simultaneous binding to ubiquitinated lysine 15 of histone H2A (H2AK15ub; not represented in the model) and di-methylated lysine of histone H4 (H4K20me2), using respectively a ubiquitin dependent recruitment domain (UDR) and a tandem tudor domain. The single disruption of one of the 2 binding sites completely abolishes 53BP1 foci formation. H2AK15ub is specifically induced at DSBs, ensuring the binding of 53BP1 exclusively at sites of damage, while H4K20me2 is present in more than 90% of the nucleosomes of non-replicated DNA, which indicates that it is not induced for the formation of 53BP1 foci. However, H4K20me2 plays a critical role for the choice of the correct DNA repair pathway depending on the replication state of DNA. In non-replicated DNA, all the nucleosomes bear H4K20me2 and thereby present the binding site for 53BP1, which in complex with RIF1 and MAD2L2, leaves no access points for BRCA1. FRAP experiments show that 80% of 53BP1 molecules at foci dynamically exchange with the nucleoplasmic pool within 30 min after photo-bleaching. In replicated DNA, H4K20me2 nucleosomes are flanked by newly synthesized nucleosomes with unmodified lysine 20 of histone H4 (H4K20me0). This opens breaches for the access of BRCA1 to the chromatin that by ubiquitination of lysine 125, 127, and 129 of histone H2A modifies the binding site for 53BP1, inhibiting the rebinding of 53BP1 from the nucleoplasmic pool and, thus, leading to its consequent release.
