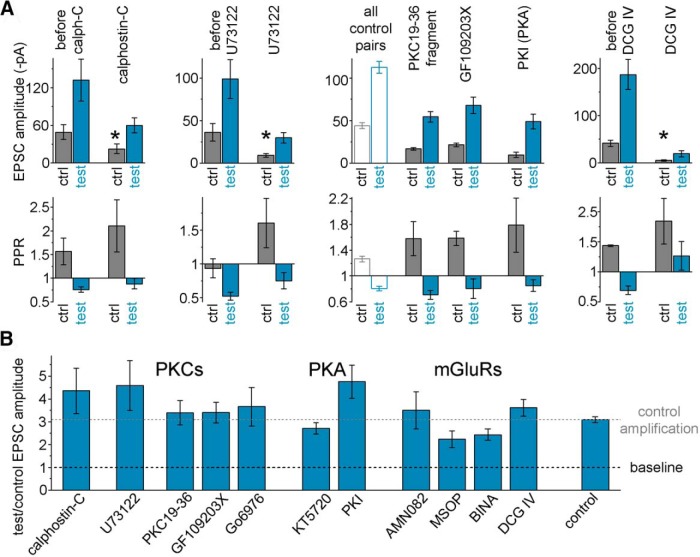
Figure 6.
Summary of the pharmacological experiments aimed at probing the potential involvement of various plasticity pathways in the postburst potentiation in FF-INs. A, Calphostin-C (1 μm, n = 12) is a PKC (and Munc13) inhibitor that acts on DAG-binding domains. U73122 (2.5 μm, n = 8) is a phospholipase C inhibitor. Both inhibitors affected baseline release as indicated by the attenuation of the control response amplitudes indicating the involvement of these pathways in the regulation of release at these synapses, serving as positive internal controls indicating drug effectiveness. PKC19-36 (100 μm, n = 7, synthetic autoinhibitory domain, applied intracellularly) and GF109203X (1 μm, n = 11, acts on the ATP binding site; slices were preincubated for at least 60 min) are selective PKC inhibitors. The small control amplitudes and large PPRs provided positive internal controls for these inhibitors. The averages of all control responses are also shown for comparison (because these inhibitors required intracellular application and pretreatment). DCG IV is an mGluR2/3 agonist (1 μm, n = 7) that selectively inhibits MF responses. * marks significant difference in the control (before burst) EPSC amplitudes in control conditions and after drug application. B, Average amplification at 1.5–6.7 s after single 15 AP bursts in the presence of various pharmacological agents. In addition to the above drugs, the burst-induced amplification of MF-EPSCs is shown in the presence of Go6976 (0.25 μm, n = 8, a subtype-selective PKC inhibitor), KT5720 (200 nm, n = 10, a PKA inhibitor that works by competing with ATP binding; PKAs are involved in pathways that regulate presynaptic release, including RIM proteins, whose PKA-dependent phosphorylation promotes vesicle priming and PKAs are also involved in posttetanic potentiation mechanisms at MF synapses onto DG inhibitory cells) (Alle et al., 2001), PKI (2.5 μm intracellularly, PKA inhibitory fragment 6–22 amide, that binds to the substrate site) (Hashimotodani et al., 2017), AMN082 (1 μm, n = 6, selective mGluR7 agonist), MSOP (150 μm, n = 8, selective inhibitor of Group III mGluRs including mGluR7), or BINA (5 μm, n = 12, selective positive allosteric modulator of mGluR2). For additional positive control effects, see Results. Postburst potentiation in the absence of these drugs (“control amplification”) is also shown for comparison. None of these pharmacological modifiers of known plasticity pathway components was able to significantly block the postburst potentiation.