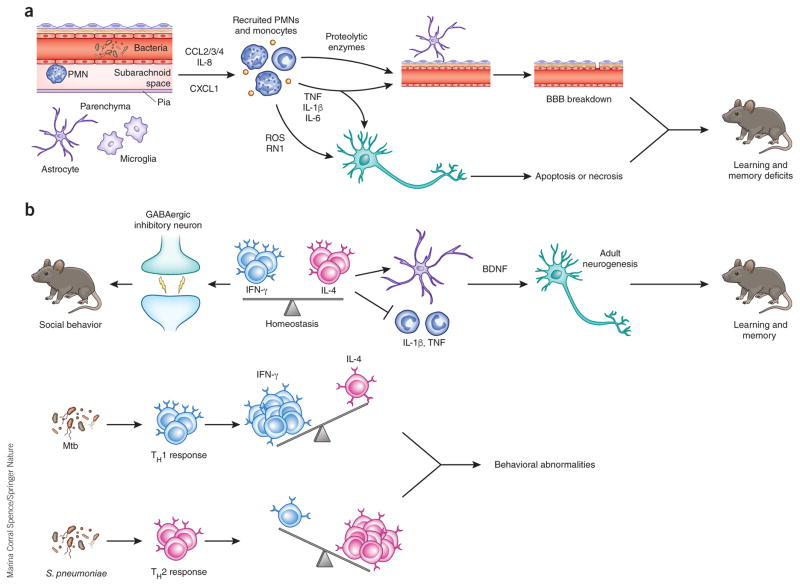
Figure 2.
Potential mechanisms of neurological sequelae subsequent to bacterial and fungal meningoencephalitis. (a) Perivascular or meningeal macrophages (PVM) recognize invading pathogens during meningoencephalitis and release a variety of chemoattractants, including CCL2, CCL3, CCL4 (CCL2/3/4), IL-8 and CXCL1, to recruit neutrophils and monocytes to the CSF compartment. Recruited neutrophils release proteolytic enzymes, which contribute to BBB breakdown through loss of tight junctions and degradation of the basement membrane. Reactive oxygen (ROS) and nitrogen (RN1) species can initiate apoptosis or necrosis in neurons. TNF, IL-1β and IL-6 can participate in BBB breakdown and neuronal death. These proinflammatory cytokines are increased in the hippocampus and cortex during experimental meningitis in mouse models and can cause learning and memory deficits in surviving animals. (b) Balance between IFN-γ and IL-4 produced by meningeal T cells is necessary for normal behavior. IFN-γ acts on inhibitory neurons to increase GABAergic current and maintain normal social behavior. IL-4 maintains normal learning and memory, potentially through inhibition of proinflammatory cytokine production by meningeal myeloid cells and/or increasing brain-derived neurotrophic factor (BDNF) production by astrocytes, which is crucial for adult neurogenesis. During infection, the balance is disrupted, which may cause behavioral alteration. Chronic infection by Mtb results in TH1 cell recruitment to the CSF compartment, increasing IFN-γ production. Meningitis caused by S. pneumoniae and other acute bacterial pathogens results in the influx of IL-4 producing TH2 cells, shifting the balance toward an IL-4-dominated cytokine milieu.