Abstract
In our literature research, we have not found any study reporting the association between the major dietary patterns and the risk of hyperuricemia in a middle-aged Chinese population. Herein, the present study aimed to evaluate the association of dietary patterns with the risk of hyperuricemia in the city of Hangzhou, Zhejiang Province, East China. We included 1204 participants (743 males and 461 females) aged 45 to 59 years in the present cross-sectional study. Dietary intake was assessed using a semiquantitative food frequency questionnaire in 2014 to 2016. All biochemical data and anthropometric measurements were collected following standardized procedures. Dietary patterns were determined by using factor analysis. We examined the associations between major dietary patterns and hyperuricemia risk by log-binominal regression analysis, and the results are presented as prevalence ratio (PR) and confidence interval (CI). Three major dietary patterns were identified by means of factor analysis: traditional Chinese, meat food, and mixed food patterns. After controlling for potential confounders, subjects in the highest quartile of the traditional Chinese pattern scores had a lower PR for hyperuricemia (PR = 0.82; 95%CI: 0.426–0.922), in comparison to those from the lowest quartile, while compared with the lowest quartile of the meat food pattern, the highest quartile had a greater PR for hyperuricemia (PR = 1.48; 95%CI: 1.120–2.097). Besides, no association was observed between mixed food pattern and the risk of hyperuricemia.
Our findings indicate that the traditional Chinese pattern is associated with a decreased risk of hyperuricemia, and the meat food pattern is associated with an increased risk of hyperuricemia, whereas the mixed food pattern shows no association with the risk of hyperuricemia. Further large prospective studies are warranted to confirm our findings.
Keywords: dietary patterns, factor analysis, hyperuricemia, middle-aged population
1. Introduction
Hyperuricemia is a purine metabolic disorder known as a precursor of gout. During the early 1980s, hyperuricemia (serum uric acid [SUA] >420 μmol/L for men, and >360 μmol/L for women) had rarely been studied in China due to its relative unimportance.[1] However, during the past several decades, with the rapid economic growth and associated lifestyle changes in China, the prevalence of hyperuricemia has increased dramatically.[2] Hyperuricemia is commonly recognized as a risk factor for some chronic diseases (e.g., diabetes, hypertension, metabolic syndrome, and chronic kidney disease).[3–6] Similarly, to our knowledge, it is also considered as a multifactorial chronic disease that may be related to some factors, including alcohol consumption, genetic and environmental factors, and especially dietary factors.[7,8]
Recent epidemiological studies reporting the association between diet and hyperuricemia have focused on the intakes of single foods, nutrients, and food components.[8–10] However, in reality, people do not eat isolated nutrients but consume meals containing many combinations of different foods and nutrients.[11] In this context, dietary pattern analysis has emerged in nutritional epidemiology as an alternative approach for examining the relationship between diet and chronic diseases, and it considers the combined effects of foods and potentially facilitate nutritional recommendations on eating practices such as healthy food choice for preventive disease.[12]
Previous studies on dietary patterns from the Chinese population have mostly reported the link with obesity, hypertension, and diabetes.[13–15] To date, only 2 epidemiological studies have reported the associations between dietary patterns and hyperuricemia risk.[16,17] Furthermore, to our knowledge, no previous study has examined the major dietary patterns in relation to hyperuricemia risk in a middle-aged Chinese population. Therefore, in this study, we aimed to identify the major dietary patterns and assess the association between dietary patterns and the risk of hyperuricemia among adults aged 45 to 59 years in China.
2. Subjects and methods
2.1. Study population
This study was carried out in Hangzhou, the capital of Zhejiang Province, east China from January 2014 to June 2016. The study sample was taken from 10 areas (Xihu, Gongshu, Shangcheng, Xiacheng, Bingjiang, Jianggan, Xiaoshan, Yuhang, Fuyang, and Linan) and 3 counties (Tonglu, Chunan, and Jiande) by a stratified cluster random-sampling method. We chose 1 residential village or community from every county or area randomly, according to resident health records, with participants aged between 45 and 59 years residing in the selected villages or communities. A total of 1353 eligible participants (743 males and 461 females) who received health examination at the Medical Center for Physical Examination, Zhejiang Hospital and Second Affiliated Hospital of Zhejiang University in 2014 and 2016 were recruited. We excluded 56 participants because of missing or incomplete dietary information in their questionnaires, and 68 participants who were taking medications for gout or hyperuricemia. Besides, we further excluded 25 participants who self-reported a family history of hyperuricemia. Finally, 1204 participants were included in our analyses. Written informed consent was obtained from all participants, and the protocol was approved by the institutional review and ethics committee of Zhejiang Hospital and the Second Affiliated Hospital of Zhejiang University.
2.2. Assessment of dietary intake
Dietary intake of 56 food items was assessed by a trained dietician using a validated, semiquantitative food frequency questionnaire (FFQ) described previously,[14] which is designed to assess average food intake over the previous year. This FFQ included foods that were frequently consumed by a middle-aged Chinese. For each food item, subjects were asked to report their average frequency of consumption over the past year and the estimated portion size, using local weight units (1 Liang = 50 g) or natural units (cups). Moreover, the frequency of each food item was classified as follows: never or occasionally, 1 to 3 times/month, 1 to 2 times/week, 3 to 4 times/week, 5 to 6 times/week, 1 time/day, 2 times/day, and 3 times/day. Then, the selected frequency category for each food item was converted to a daily intake and used in the further analysis.
2.3. Identification of dietary patterns
The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and the Bartlett's test of sphericity were used to evaluate the adequacy of correlation matrices with the data. Factor analysis (principal component) was used to derive the major dietary patterns based on the frequency of consumption of 56 food groups in this FFQ. In this method, all variables were considered simultaneously, each one related to the others. The factors were rotated using varimax rotation to achieve orthogonal (uncorrelated) factors, which are easier to interpret. Factor loadings (e.g., measurements of correlations between each variable and the factors) were analyzed. The higher the factor loading of a food group, the greater the contribution of that group to the pattern. In determining the number of factors to retain, the eigenvalue and scree plot were applied.[18] In our analyses, factors with Eigen values ≥1.5 were extracted and then scree plots were used to identify the major dietary patterns. Labeling of dietary patterns was based on the interpretation of foods with high factor loadings for each dietary pattern.[19] A factor score obtained for each participant was calculated by summing the consumption of each food group that were weighted by factor loading, the higher score showing intake of more food groups associated with that respective pattern. Finally, only food groups with absolute factor loading ≥0.3 were considered to be important contributors to this pattern and included in the present study.
2.4. Assessment of biomarker
A blood sample was drawn between 7:00 and 9:00 in the morning into evacuated tubes after fasting overnight (12 h). After blood samples were taken, serum was separated by centrifugation for 10 minutes at 3000 rpm. Then samples were analyzed in the Medical Center for Physical Examination, Zhejiang Hospital and the Second Affiliated Hospital of Zhejiang University for fasting plasma glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, SUA, alanine aminotransferase, and asparagine aminotransferase by using an autoanalyzer (the Hitachi 7180 auto-analyzer, Tokyo, Japan).
2.5. Assessment of other variables
Data about physical activity were obtained by using a validated self-reported questionnaire[14] and expressed as metabolic equivalents in hours per week (MET-h/week). Information on smoking status was collected and categorized into never smokers, current smokers, and former smokers. The educational level was classified as follows: primary school or below, middle and high school, and junior college or above. Total energy intake was estimated through the semiquantitative FFQ, expressed in kilocalorie per day (kcal/day).
2.6. Assessment of blood pressure
For blood pressure measurements, subjects were first asked to rest for 10 minutes. Then, a well-trained nurse measured blood pressure using a standard mercury sphygmomanometer with the subjects in the sitting position, and thereafter the mean of 3 measurements was considered as the subject's blood pressure in our analyses.
2.7. Definition of terms
Hyperuricemia was defined as SUA ≥420 μmol/L (7.0 mg/dL) for men, and ≥360 μmol/L (6.0 mg/dL) for women.[1] Body mass index (BMI) was calculated as weight (kilogram)/height (meter)2. Waist circumstance (WC) was measured at the end of normal expiration in duplicate on bare skin midway between the lowest rib and the superior border of the iliac crest.[14] Blood pressure was measured by using an automated sphygmomanometer with the subjects in sitting position. Obesity was defined by BMI ≥28 kg/m2 and abdominal adiposity was defined as a WC ≥85 cm for men and ≥80 cm for women in a Chinese population.[20] Hypertension was defined as a systolic pressure of ≥140 mm Hg and/or a diastolic pressure of ≥90 mm Hg.[21]
2.8. Statistical analyses
Quartiles based on factor scores were determined for each dietary pattern (the highest category and the lowest category represented high and low intake of each dietary pattern, respectively). The characteristics of study participants were calculated across quartiles of each dietary pattern score. The data for continuous variables were generally reported as the mean ± SD, and the data for categorical variables were reported as sum (percentages). The χ2 test was used to assess the difference for categorical variables, while the analysis of variance (ANOVA) was used to describe mean differences for continuous variables. Age was included as a continuous variable. The potential confounding variables that were considered were gender (male/female), age (continuous), education level (<high school, high school, and >high school), physical activity level (light, moderate, and heavy), smoking status (never, current, and former), alcohol use (g/d), hypertension (yes/no), BMI (continuous), and total energy intake (kcal/d). After adjustment for potential confounders, log-binominal regression analysis was used to assess the relation between dietary patterns and hyperuricemia risk. All statistical analyses were carried out with the use of the SPSS software package version 20.0 for Windows (SPSS Inc, Chicago, IL), and a 2-tailed P <.05 was considered significant.
3. Results
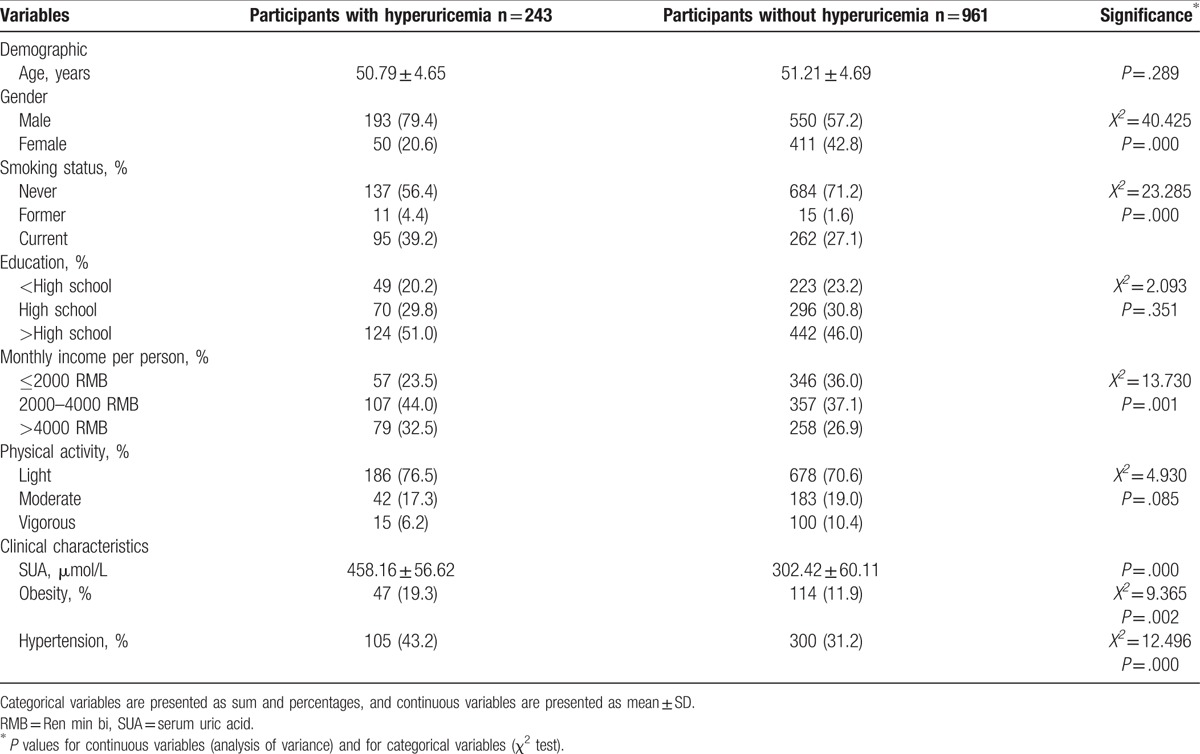
Overall prevalence of hyperuricemia in this population was 20.2%, with male was 16.0% and female was 4.2%. The demographic and clinical characteristics of participants according to with and without hyperuricemia are shown in Table 1. There were significant differences between participants with and without hyperuricemia by gender, smoking status, economic income, SUA level, obesity, and hypertension.
Table 1.
Demographic and clinical characteristics of participants in the Hangzhou Nutrition and Health Study.
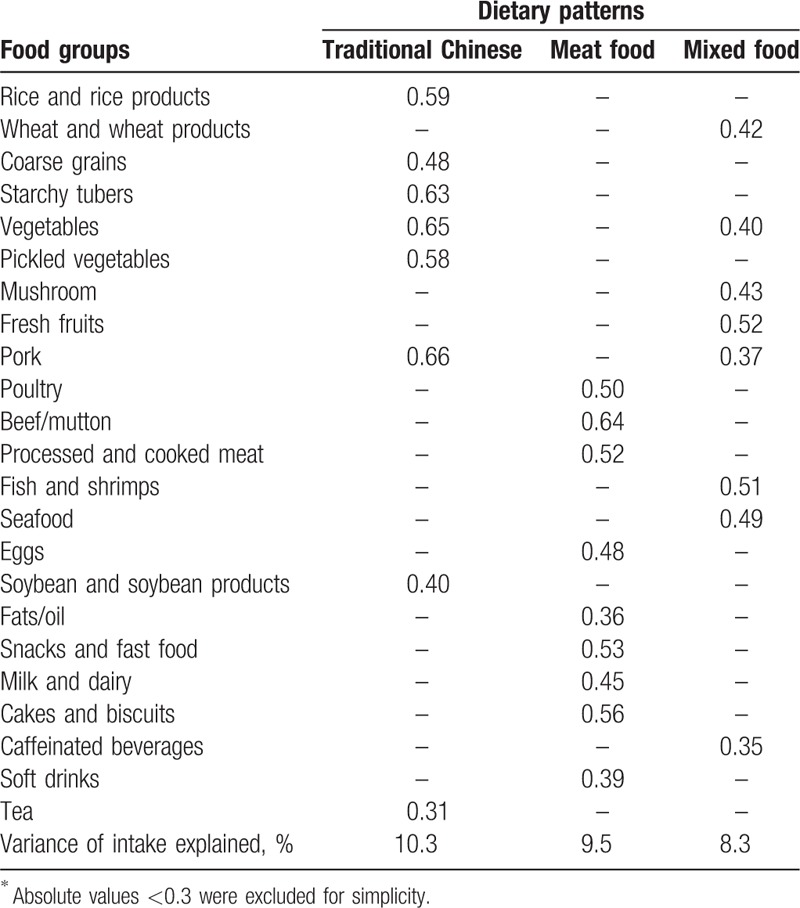
Both the Kaiser–Meyer–Olkin index (0.755) and Bartlett's test (P <.001) showed that the correlation among the variables was sufficiently strong for a factor analysis.[19] Factor analysis revealed 3 major dietary patterns. The first, labeled traditional Chinese dietary pattern was loaded by a high intake of rice and rice products, coarse grains, starchy tubers, vegetables, pickled vegetables, pork, soybean and soybean products, and tea. The second, labeled meat food dietary pattern was characterized by high intakes of poultry, beef/mutton, processed and cooked meat, eggs, fats/oil, snacks and fast food, milk and dairy, cake and biscuits, and soft drinks. The third, labeled mixed dietary pattern was characterized by high intakes of wheat and wheat products, vegetables, mushroom, fresh fruits, pork, fish and shrimps, seafood, and caffeinated beverages. Overall, these 3 factors explained 28.1% of the entire variance. Moreover, the factor-loading matrixes for 3 dietary patterns are presented in Table 2.
Table 2.
Factor-loading matrix for 3 major dietary patterns among 1204 Chinese adult aged 45 to 59 years∗.
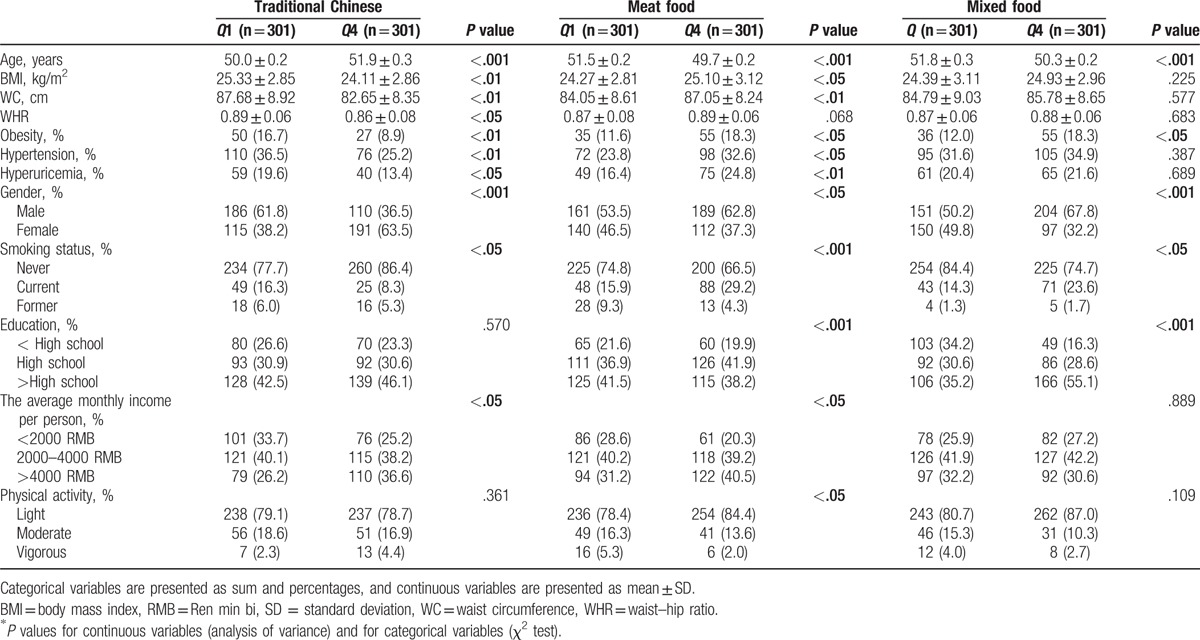
The characteristics of the study participants by quartile (Q) categories of dietary pattern scores in Hangzhou are shown in Table 3. Compared with participants in the lowest quartile, those in the highest quartile of the traditional Chinese dietary pattern were more likely to be female, older, nonsmokers, and had lower prevalence of obesity, hypertension and hyperuricemia, lower BMI, WC, and waist–hip ratio, and higher income. Besides, in comparison with the participants from the lowest quartile of the meat food dietary pattern, those in the highest quartile were more likely to be younger, male, smokers, and had higher prevalence of obesity, hypertension and hyperuricemia, and higher BMI and WC. Similarly, participants in the highest quartile of the mixed dietary pattern were more likely to be younger, male, smokers with higher education level and prevalence of obesity than those in the lowest quartile.
Table 3.
Characteristics of the study participants by quartile (Q) categories of dietary pattern scores in Hangzhou.
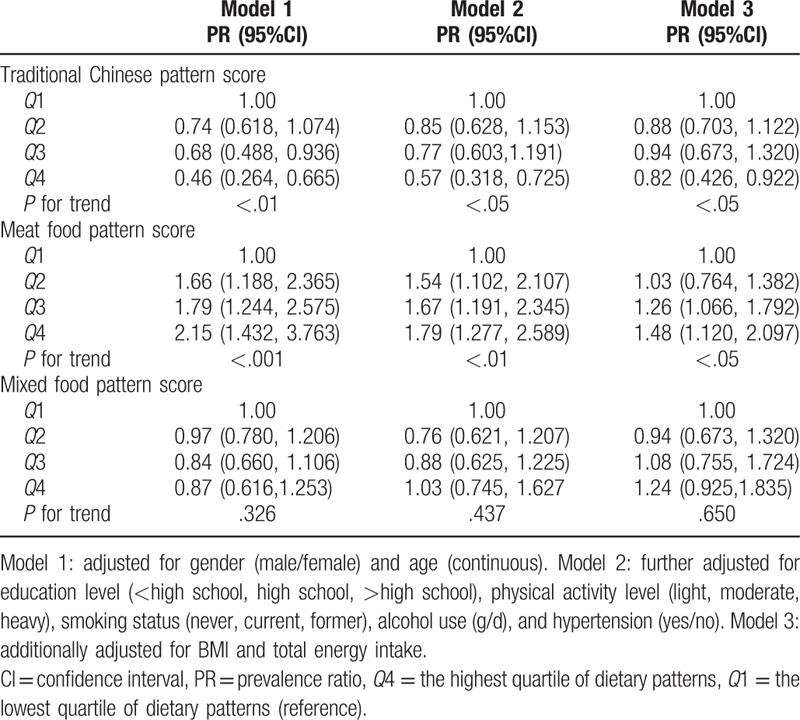
The association between dietary patterns and the risk of hyperuricemia by log-binomial regression is presented in Table 4. After controlling for potential confounders, participants in the highest quartile of the traditional Chinese dietary pattern scores had lower prevalence ratio (PR) for hyperuricemia (PR = 0.82; 95% confidence interval [CI]: 0.426–0.922, P <.05) than did those in the lowest quartile, whereas those in the highest quartile of the meat food dietary pattern score had greater PR for hyperuricemia (PR = 1.48; 95% CI: 1.120–2.097, P <.05) than did those in the lowest quartile. No statistically significant association was observed between the mixed food pattern and hyperuricemia risk.
Table 4.
Multivariable adjusted PR (95%CI) for hyperuricemia across the quartile (Q) categories of dietary pattern scores in Zhejiang Province, China.
4. Discussion
In this study, we derived 3 major dietary patterns by means of factor analysis: the traditional Chinese, the meat food, and the mixed patterns. The results of this study indicate that the traditional Chinese pattern is associated with a decreased risk of hyperuricemia, whereas the meat food pattern is associated with an elevated risk of hyperuricemia among a Chinese population aged 45 to 59 years. Besides, no significant association is found between the mixed dietary pattern and hyperuricemia risk, even after adjusting for potential confounders. To the best of our knowledge, this is the first study in a middle-aged Chinese population to examine the association of major dietary patterns with the risk of hyperuricemia.
In our analyses, consumption of a traditional Chinese dietary pattern, characterized by high intake of rice and rice products, coarse grains, starchy tubers, vegetables, pickled vegetables, pork, soybean and soybean products, and tea, was associated with a decreased risk of hyperuricemia. Compared with those in the lowest quartile of the traditional Chinese pattern scores, participants in the highest quartile of intake had a lower PR for hyperuricemia (13.4% vs 19.6%). Our findings are consistent with prior studies, suggesting that the healthy dietary pattern is associated with a decreased risk of hyperuricemia.[16] Zhang et al[16] reported decreased odds of hyperuricemia in Chinese adults who scored higher on a “soybean products and fruit” pattern (high consumption of soybean products, fruits, vegetables, and starchy tubers). In their analyses, the results showed that OR for the top tertile of score for the “soybean products and fruit” pattern was 0.32 (95%CI: 0.19–0.57) compared with the lowest tertile of the “soybean products and fruit” pattern score. One possible mechanism of their apparently protective effect against hyperuricemia is that they are good sources of antioxidants (e.g., vitamin C, vitamin E, and other carotenoids compounds), isoflavones, and dietary fiber. First, dietary fiber has been recognized as having a potential role in binding uric acid in the gut for excretion.[22] Some previous studies have also indicated that dietary fiber intake is inversely associated with the risk of hyperuricemia.[9] Second, fruits and vegetables in the traditional Chinese pattern contain large amounts of vitamin C, which has been shown to reduce oxidative stress and inflammation to lower uric acid synthesis.[23] Stein et al[24] reported that vitamin C has uricosuric properties, increasing renal fractional clearance of uric acid, thereby reducing SUA. Besides, previous a meta-analysis of vitamin C and serum uric acid concluded that vitamin C supplementation significantly lowered SUA level.[25] Third, evidence from epidemiological and experimental studies indicated that drinking green tea was inversely associated with hypertension, which is an important risk factor for hyperuricemia.[26] Finally, greater soybean and its products consumption was associated with a lower presence of hyperuricemia in women.[27] The inverse association of soy food with hyperuricemia might have been partly ascribed to isoflavones, which may inhibit the xanthine oxidase, oxidizing hypoxanthine and xanthine to uric acid in the purine catabolic pathway.[28]
The meat food pattern, characterized by high intake of poultry, beef/mutton, processed and cooked meat, eggs, fats/oil, snacks and fast food, milk and dairy, cake and biscuits, and soft drinks, was associated with an increased risk of hyperuricemia. Our findings are consistent with a previous study suggesting that animal food, for example, meat and seafood consumption, found among populations participating in the Health Professionals Follow-up Study, was significantly associated with an increased risk of gout.[29] In the Health Professionals Follow-up Study, Choi et al[29] found that the multivariate relative risk of gout among men in the highest quintile of meat intake, as compared with those in the lowest quintile, was 1.41 (95%CI: 1.07–1.86; P for trend = .02).The unfavorable effect of animal food pattern could be attributable to this pattern's unhealthy constituents (e.g., meat, seafood, and soft drinks). Meat and seafood often contain a high content purine, which is positively associated with the risk of hyperuricemia.[30,31] In addition, high consumption of meat has been associated with an increased risk of obesity.[14] Epidemiological studies have demonstrated a strong correlation between obesity and hyperuricemia.[32,33] Furthermore, soft drinks contain large amount of fructose, which can play a role as a source of intracellular uric acid production and results in increased serum uric acid level.[34] Some epidemiological studies have suggested that fructose intake may contribute to increased risk of hyperuricemia.[35,36]
The mixed food pattern was characterized by high intake of wheat and wheat products, vegetables, mushroom, fresh fruits, pork, fish and shrimps, seafood, and caffeinated beverages. In the present study, we found no significant association between this pattern and hyperuricemia, though the prevalence of hyperuricemia for the highest category of this pattern was higher compared with the lowest category (21.6% vs 20.4%). The complex nature of this pattern may explain this finding to some extent. On the one hand, fruits and vegetables in the mixed dietary pattern are considered as a source of antioxidants such as vitamin C. Previous studies have found that vitamin C is associated with a decreased risk of hyperuriemia.[24,25] Besides, evidence from epidemiological and experimental studies indicated that drinking green tea was inversely associated with obesity, which is an important risk factor for hyperuricemia.[37] On the other hand, as mentioned above, high consumption of meat was associated with an increased risk of hyperuricemia.[30] Finally, a null association between mixed pattern and hyperuricemia could also be due to reverse causality. Participants with risk of hyperuricemia may modify their dietary habits to reduce the intake of high purine food during a routine examination. In a word, these possibilities could not be excluded in this study.
4.1. Strengths and limitations
The present study had strengths and limitations. First, to the best of our knowledge, this is the first study in middle-aged Chinese to assess the association between dietary patterns and hyperuricemia risk. Second, information about dietary intake were collected by trained dieticians during a structured interview, using a validated semiquantitative FFQ. Thus, our results are reliable. Third, we have controlled for several potential known confounding factors for reliability in our analyses. Nevertheless, some limitations of this study need to be acknowledged. First, the main limitation of the present study is its cross-sectional nature, which prevented us from making a causal inference. Thus, our findings need to be confirmed in the future prospective study. Second, the use of principal component analysis requires several subjective decisions in the selection of included variables as well as in the detainment of number of factors to retain.[38] Third, the measurement errors in reporting diet using the FFQ affected our results by introducing random variation and lowering the significance of the associations. Besides, although we adjusted for multiple potential confounding variables in the multivariable-adjusted model, we were unable to control the effect of unmeasured confounders (e.g., use of diuretics) or residual confounding. Finally, the study participants were volunteers recruited in the city of Hangzhou and not members of a random sample. Also, these volunteers tend to have higher income and educational level than the general population. Thus, the generalizability of our results to the population level was limited.
5. Conclusions
In conclusion, we identified 3 major dietary patterns: traditional Chinese, meat food, and mixed patterns. Our results indicate that the traditional Chinese pattern is associated with a decreased risk of hyperuricemia, whereas the meat food pattern is associated with an elevated risk of hyperuricemia. These findings are important for developing interventions and policies addressing hyperuricemia prevention among a middle-aged Chinese population. However, this result should be interpreted with caution because of its limitations. Further prospective studies are needed to prove these findings.
Acknowledgments
The authors thank all participants from the Department of Nutrition, Zhejiang Hospital and the second affiliated hospital of Zhejiang University for their assistance and support. They also acknowledge the Medical Center for Physical Examination, Zhejiang Hospital and the Second Affiliated Hospital of Zhejiang University for important contributions to collection of data in this study.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, CI = confidence interval, FFQ = food frequency questionnaire, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, PR = prevalence ratios, SUA = serum uric acid, TC = total cholesterol, TG = triglyceride, WC = waist circumference.
Authors’ contributions: FH and XLY conceived and designed the experiments. HF and LLW conducted research. XLY analyzed data and wrote the paper. All authors read and approved the final manuscript.
Funding: This study was supported by the medical platform projects of Zhejiang Province (grant no. 2016ZDA001) and Natural Science Foundation of Zhejiang (grant no. Y17H030031).
The authors have no conflicts of interest to disclose.
References
- [1].Liu L, Lou S, Xu K, et al. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin Rheumatol 2013;32:233–9. [DOI] [PubMed] [Google Scholar]
- [2].Zhang Q, Lou S, Meng Z, et al. Gender and age impacts on the correlations between hyperuricemia and metabolic syndrome in Chinese. Clin Rheumatol 2011;30:777–87. [DOI] [PubMed] [Google Scholar]
- [3].Sluijs I, Beulens JW, van Der ADL, et al. Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J Nutr 2013;143:80–5. [DOI] [PubMed] [Google Scholar]
- [4].Grayson PC, Kim SY, LaValley M, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442–7. [DOI] [PubMed] [Google Scholar]
- [6].Filiopoulos V, Hadjiyannakos D, Vlassopoulos D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren Fail 2012;34:510–20. [DOI] [PubMed] [Google Scholar]
- [7].Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 2004;363:1277–81. [DOI] [PubMed] [Google Scholar]
- [8].Yu KH, See LC, Huang YC, et al. Dietary factors associated with hyperuricemia in adults. Semin Arthritis Rheum 2008;37:243–50. [DOI] [PubMed] [Google Scholar]
- [9].Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyper-uricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 2008;35:1859–64. [PubMed] [Google Scholar]
- [10].Villegas R, Xiang YB, Elasy T, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr Metab Cardiovasc Dis 2012;22:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schulze MB, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br J Nutr 2006;95:860–9. [DOI] [PubMed] [Google Scholar]
- [12].Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- [13].Zheng PF, Shu L, Zhang XY, et al. Association between dietary patterns and the risk of hypertension among Chinese: a cross-sectional study. Nutrients 2016;8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shu L, Zheng PF, Zhang XY, et al. Association between dietary patterns and the indicators of obesity among Chinese: a cross-sectional study. Nutrients 2015;7:7995–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frank LK, Kröger J, Schulze MB, et al. Dietary patterns in urban Ghana and risk of type 2 diabetes. Br J Nutr 2014;112:89–98. [DOI] [PubMed] [Google Scholar]
- [16].Zhang M, Chang H, Gao Y, et al. Major dietary patterns and risk of asymptomatic hyperuricemia in Chinese adults. J Nutr Sci Vitaminol (Tokyo) 2012;58:339–45. [DOI] [PubMed] [Google Scholar]
- [17].Tsai YT, Liu JP, Tu YK, et al. Relationship between dietary patterns and serum uric acid concentrations among ethnic Chinese adults in Taiwan. Asia Pac J Clin Nutr 2012;21:263–70. [PubMed] [Google Scholar]
- [18].Zhang C, Schulze MB, Solomon CG, et al. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetoloqia 2006;49:2604–13. [DOI] [PubMed] [Google Scholar]
- [19].Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- [20].Wang HJ, Wang ZH, Yu WT, et al. Changes of waist circumference distribution and the prevalence of adiposity among Chinese adults from 1993 to 2006. Eup Pubmed Cent 2008;29:953–8. [PubMed] [Google Scholar]
- [21].Zhang J, Zhang K, Shi H, et al. A cross-sectional study to evaluate the associations between hypertension and osteoporosis in Chinese postmenopausal women. Int J Clin Exp Med 2015;8:21194–200. [PMC free article] [PubMed] [Google Scholar]
- [22].Sorensen LF. Gout secondary to chronic renal disease: studies on urate metabolism. Ann Rheum Dis 1980;39:424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequency of megavitamin therapy. Ann Intern Med 1976;84:385–8. [DOI] [PubMed] [Google Scholar]
- [25].Juraschek SP, Miller ER, 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2011;63:1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li W, Yang J, Zhu XS, et al. Correlation between tea consumption and prevalence of hypertension among Singaporean Chinese residents aged ≥40 years. J Hum Hypertens 2016;30:11–7. [DOI] [PubMed] [Google Scholar]
- [27].Liu J, Sun LL, He LP, et al. Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr Metab Cardiovasc Dis 2014;24:1097–104. [DOI] [PubMed] [Google Scholar]
- [28].Li Y, Frenz CM, Li Z, et al. Virtual and in vitro bioassay screening of phytochemical inhibitors from flavonoids and isoflavones against xanthine oxidase and cyclooxygenase-2 for gout treatment. Chem Biol Drug Des 2013;81:537e44. [DOI] [PubMed] [Google Scholar]
- [29].Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093–103. [DOI] [PubMed] [Google Scholar]
- [30].Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005;52:283–9. [DOI] [PubMed] [Google Scholar]
- [31].Chuang SY, Lee SC, Hsieh YT, et al. Trends in hyperuricemia and gout prevalence: Nutrition and Healthy Survey in Taiwan from 1993–1996 to 2005–2008. Asia Pac J Clin Nutr 2011;20:301–8. [PubMed] [Google Scholar]
- [32].Chen Y, Zhang N, Sun G, et al. Metabolically healthy obesity also has risk for hyperuricemia among Chinese general population: a cross-sectional study. Obes Res Clin Pract 2016;10(suppl 1):S4–95. [DOI] [PubMed] [Google Scholar]
- [33].Roubenoff R, Klag MJ, Mead LA, et al. Incidence and risk factors for gout in white men. JAMA 1991;266:3004–7. [PubMed] [Google Scholar]
- [34].Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 2009;30:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Choi JW, Ford ES, Gao X, et al. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008;59:109–16. [DOI] [PubMed] [Google Scholar]
- [36].Bae J, Chun BY, Park PS, et al. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: the Korean Multi-Rural Communities Cohort Study. Semin Arthritis Rheum 2014;43:654–61. [DOI] [PubMed] [Google Scholar]
- [37].Rocha A, Bolin AP, Cardoso CA, et al. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr 2016;55:2231–44. [DOI] [PubMed] [Google Scholar]
- [38].Chan R, Chan D, Woo J. Associations between dietary patterns and demographics, lifestyle, anthropometry and blood pressure in Chinese community-dwelling older men and women. J Nutr Sci 2012;1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]


