Abstract
Stereotactic body radiation therapy (SBRT) for inoperable hepatocellular carcinoma (HCC) offers excellent local control rates. This study retrospectively analyzed the influence of different tumor size on treatment outcomes after SBRT.
Between December 2008 and February 2014, 141 HCC patients were treated with Cyberknife SBRT. Patients were divided into 3 groups namely small tumors (≤4 cm), intermediate-sized (>4–<10 cm), and large (≥10 cm) tumors. Treatment outcomes, prognoses, and safety at each tumor size were compared and analyzed.
A total of 52 patients with small tumors, 55 with intermediate tumors, and 34 patients with large tumors were retrospectively analyzed with a median follow-up of 16 months. Objective responses were achieved at 96.15%, 90.90%, and 76.47% for small, intermediate, and large tumors, respectively (P ≤ .0001) and the 3-year local control rates were 97.85%, 71.99%, and 82.14%, respectively (P = .0035). The 3-year overall survival rates were 50.26%, 45.29%, and 33.38% for small, intermediate, and large tumors, respectively (P = .3757). No significant differences were found in overall-survival, intra-hepatic recurrence free survival, disease-progression free survival, or distant metastasis-free survival.
SBRT offers the best effective local control rate and response rate for small HCCs. However, tumor size did not significantly affect the overall survival rate, intra-hepatic recurrence free rate, or disease-progression free rate.
Keywords: cyberknife stereotactic body radiation therapy, hepatocellular carcinoma, tumor size
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and constitutes the third most common cause of cancer-related death globally. Surgical resection, liver transplant, and radiofrequency ablation (RFA) for tumors ≤3 cm are the only curative treatments.[1–3] However, a substantial number of HCC patients remain ineligible for these standard treatments. Transarterial chemoembolization (TACE) is the most common alternative, but its local control rate is inferior to that obtained using resection and RFA. The 3-year local control rate from superselective TACE has been reported to be 65% for patients with HCCs <5 cm in diameter.[4]
Due to recent advances in computer and imaging technologies, stereotactic body radiation therapy (SBRT) has become a safe and feasible technique for HCC patients with radiation-induced liver disease (RILD) rates of ≤5%. SBRT has emerged as a treatment option for HCC patients who are ineligible for surgery, RFA, or liver transplant. Studies in HCC patients treated with SBRT have shown high local control rates of 70% to 100%.[5–8]
Until recently, the published literature rarely mentioned outcomes after SBRT based on different tumor sizes. In RFA and transcatheter arterial embolization (TAE), tumor size was proven to be a major independent predictor of survival and local control rate. HCCs <5 cm tended to respond favorably after treatment with either RFA or TAE.[9–11] Larger tumors are at greater risk, when using SBRT, not only for loss of local control and toxicity due to larger irradiated volumes, but regional and distant failures as well. However, it is still unknown whether a tumor size threshold exists beyond which local control is compromised. In addition, it is still unclear whether SBRT of smaller HCCs would produce better outcomes than treatment of larger tumors.
This study reviewed a single institution's experience using SBRT in treating inoperable HCC with the aim to examine the impact of tumor size on local control, overall survival, intrahepatic recurrence free rate, and toxicity after SBRT.
2. Methods and materials
2.1. Patient selection
Written informed consent was obtained from all patients before treatment and the study was approved by the Institutional Review Board of Chi Mei Medical Center.
Using research ethics board approved single institution protocol, 141 inoperable HCC patients were treated with Cyberknife SBRT between December 2008 and February 2014.
Patients were included in our study based on the following criteria: pathological confirmation of HCC; at least 1 radiological image with the classic HCC feature of enhancement accompanied by a level of serum tumor marker alpha fetoprotein (AFP) >200 ng/mL or at least 2 radiological images (CT/MRI/Angiogram) with the classic imaging findings of HCC; patients with unresectable, medically inoperable HCC; an ECOG performance status ≤2.
Patients with multiple extrahepatic metastases, those with previous radiation therapy of the liver, SGOT and SGPT levels ≥2.5 times higher than the upper limit, a Child-Pugh score ≥7, intractable ascites, tumor closely attached to esophagus, stomach, duodenum and bowel, and a normal liver volume <700 cm3 were excluded from treatment.
Mandatory baseline examinations included dynamic magnetic resonance imaging (MRI) and/or triphasic computed tomography (CT) of the liver, complete blood chemistry, liver function tests, hepatitis B and C antigens and viral titers, AFP level, and chest x-ray images. Patients with positive hepatitis B surface antigen (HbsAg) or elevated hepatitis B viral titers were given prophylactic anti-retroviral therapy from the start of SBRT to at least 6 months after treatment for prevention of post-RT reactivation of HBV.[12]
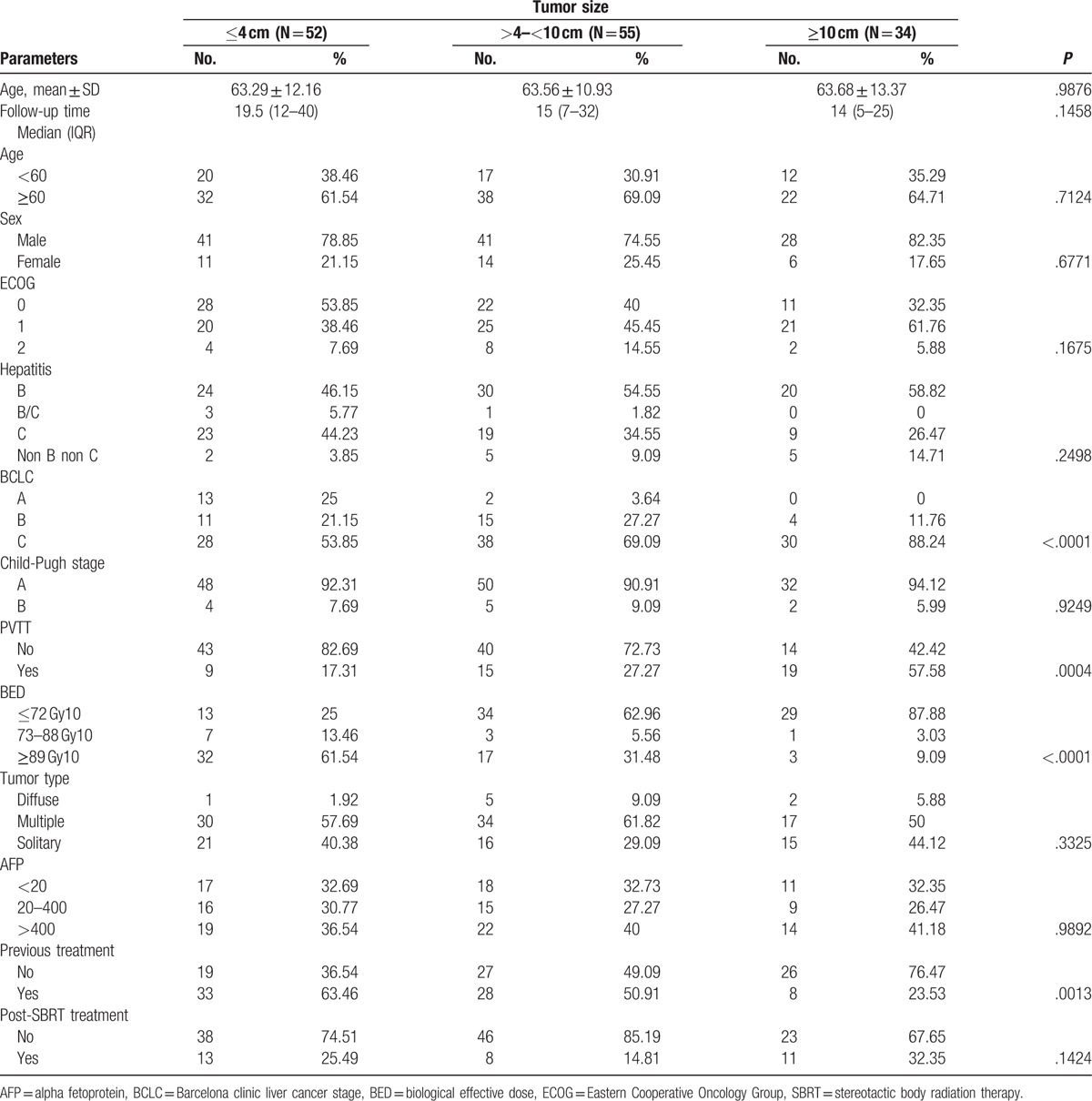
The characteristics and disease variables of the 141 patients divided into 3 specific groups namely small tumors (≤4 cm), intermediate-sized (>4–<10 cm), and large (≥10 cm) tumors at the time of radiation treatment are summarized in Table 1. The patients’ ages ranged from 31 to 91 years with a median age of 66 years and male gender predominated. Median follow-up time was 16 months (range, 2–72 months).
Table 1.
Patient characteristics (total no. = 141).
3. Treatment details
3.1. SBRT
SBRT was performed using the Cyberknife, a robotic image-guided whole body radiosurgical system equipped with the synchrony system (i.e., a real-time respiratory tracking system for target volumes that move with respiration. The contouring was performed on the planning CT with contrast. All patients were positioned on individually shaped vacuum pillows and wore a treatment jacket on which the optical markers were fixed. Any displacement of the patient during treatment was detected by either internal or external fiducial markers with sub-millimeter accuracy.[13]
3.2. Dose specification and plan evaluation
Prescribed doses, doses per fraction, and number of fractions were individualized based upon tumor size, location, amount of normal liver available, and organ at risk. SBRT doses ranged from 26 to 40 Gy in 5 fractions, with 26 to 40 Gy in 3 to 5 fractions given to tumors >5 cm and 39 Gy in 3 fractions to tumors ≤5 cm. Tumor maximum diameter ranged from 1.8 to 18 cm.
The SBRT doses were converted to the normalized total dose at a fraction size of 2 Gy (NTD2 Gy) using a linear quadratic equation (BED = total dose × [1 + dose per fraction/α/β], α/β = 10 for early responding tissue, α/β = 3 for late responding tissue). NTD2 Gy (α/β = 10) of SBRT ranged from 48.36 to 89.70 Gy. The gross tumor volume (GTV) included contrast-enhancing disease visible on CT scan or MRI with contrast. No additional clinical target volume (CTV) was added. The GTV was directly expanded 1 to 3 mm in all directions to create the planning target volume (PTV). Modification of PTV was done if it extended into the dose-limiting organs, except the normal liver. Radiation doses were prescribed to the isodose line ranging from 59.9% to 96.9% of the maximum dose and the median isodose line was 79.93%. Treatment was delivered via the real-time tracking system using the fiducial as a guide; planning was performed with the MultiPlan Cyberknife Treatment Planning System version 2.10.
The protocol dose constraints for normal liver (total liver minus cumulative GTV) specified that a minimum volume of 700 mL should receive a total dose <15 Gy in 3 fractionations and 18 Gy in 5 fractionations; 66.7% of the ipsilateral right kidney volume should be <15 Gy in 3 fractionations and 18 Gy in 5 fractionations. The maximum total dose to any point in the spinal cord should not exceed 18 Gy in 3 fractionations and 21 Gy in 5 fractionations, and stomach, bowel, duodenum, heart should not exceed 30 Gy in 3 fractionations and 35 to 38 Gy in 5 fractionations, while the esophagus should not receive >27 Gy. Efforts were made to minimize the dose to all normal tissues as much as possible.
3.3. Follow-up, response, and toxicity assessment
After completion of treatment, patients were then followed-up every 1 to 2 weeks in the first month and every 3 months thereafter. AFP levels and imaging studies (with either 4-phase CT scanning or dynamic MRI of liver) were performed every 1 to 2 months and subsequently every 3 to 4 months.
Toxicity grading was performed according to Common Toxicity Criteria Adverse Events version 4.0. Acute toxicities were defined as adverse events occurring within 3 months after SBRT, and late toxicities were those occurring after 3 months. Radiation-induced liver disease was defined as either classic or non-classic RILD. Classic RILD was characterized as the presence of nonmalignant ascites and anicteric elevation of alkaline phosphatase level twice the upper level of normal or baseline value occurring between 2 weeks and 3 months after the completion of irradiation. Non-classic RILD, typically occurring between 1 week and 3 months after therapy, involves elevation of transaminase to at least 5 times the upper limit of the normal or pretreatment level within 4 months of irradiation completion or decline in liver function in the absence of classic RILD.[14] This endpoint was common in HCC patients with poor liver function (i.e., hepatitis B infection, Child-Pugh Classes B and C). The diagnoses of both types of RILD could be made only in the absence of evidence of tumor progression. Toxicity grading was recorded based on the worst toxicity recorded.
Tumor response was assessed using response evaluation and criteria in solid tumors (RECIST). Complete disappearance of the tumor was defined as a complete response (CR), and a partial response (PR) was defined as a decrease of >30% in the longest diameter of the target tumors. A decrease of <30% or no change was defined as stable (SD), and progression of >20% was defined as progressive disease (PD). Local control was defined as being free from the development of a new lesion or an increase in tumor size within the PTV. Free from intrahepatic recurrence was defined as being free from the development of a new lesion in the liver outside the PTV. Distant metastasis was defined as recurrence beyond the liver. Disease progression was defined as the development of intrahepatic recurrence and distant metastasis.
3.4. Statistical analysis
Patient characteristics in all 3 groups (tumor size ≤4 cm, >4–<10 cm, ≥10 cm) were compared with Mann–Whitney U or t tests and Fisher exact for continuous and categorical variables, respectively. Overall survival curves and local control rates were calculated using Kaplan–Meier analysis. Log-rank testing was used to compare outcomes between the subsets of patient analyzed. For all tests, two-sided P-values <.05 were considered significant. Data were analyzed with SPSS statistics.
4. Results
4.1. Patient characteristics
Patients were divided into 3 groups based on tumor size (i.e., ≤4 cm, >4–<10 cm, ≥10 cm). Relevant treatment and tumor characteristics are shown in Table 1. Characteristics among the 3 groups of patients were comparable. The proportion of patients with small tumors (≤4 cm), intermediate tumors (>4–<10 cm), and large tumors (≥10 cm) were 36.88% (52 patients), 39% (55 patients), and 24.11% (34 patients), respectively. Median follow-up durations in small, intermediate, and large tumor groups were 19.5 months (range, 12–40 months), 15 months (range, 7–32 months), and 14 months (range, 5–25 months), respectively. The proportion of Barcelona clinic liver cancer stage (BCLC) C patients were significantly higher in all groups, while BCLC A was relatively higher in the small tumor group (25%) compared with the intermediate (3.64%) and large tumor groups (0%). Patients without portal vein tumor thrombosis were significantly higher in small (82.69%) and intermediate tumor size group (72.73%), while large tumor group had a larger numbers of patients with portal vein tumor thrombosis (57.58%). Other significant differences among the 3 groups included the finding that smaller tumors (≤4 cm) were more likely to be treated with higher bioequivalent dose (BED) regimens (i.e., 39 Gy/3 fractions) because of the larger amount of normal liver volume available, while for larger tumors (because of dose-constraints due to the smaller liver volume available), a lower dose (40–45 Gy/5 fractions) with a much lower BED was prescribed.
4.2. Treatment outcomes
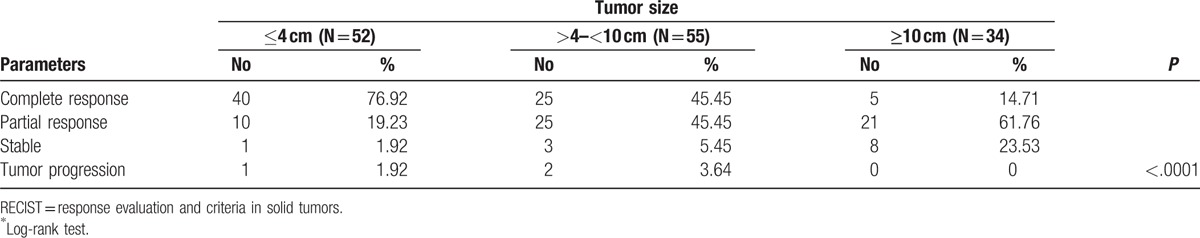
Tumor response rates are shown in Table 2. Objective response (CR + PR) was achieved at 96.15%, 90.90%, and 76.47% for small, intermediate, and large tumors, respectively (P ≤ .0001). The complete response rate was significantly higher (i.e., 40 patients [76.92%]) for small tumor size, versus 25 patients (45.45%) for the intermediate tumor group, and 5 patients (14.71%) the large tumor group. During the follow-up period, local tumor progression was found in 1 patient (1.92%) in the small tumor group and 2 patients (3.64%) in the intermediate tumor group. The 1-, 2-, and 3-year overall survival rates were 76.92%, 55.85%, and 50.86% for the small tumor group, 60.09%, 51.32%, 45.29% for the intermediate tumor group, and 55.88%, 38.95%, 33.38% for large tumor group, respectively. For the small tumor group, the 1-, 2-, 3-year disease-progression free rates were 52.63%, 42.03%, and 39.39%, respectively; for the intermediate tumor group, they were 48.84%, 33.33%, and 31.65%, respectively, and for the large tumor group, the 1-, 2-, 3-year disease-progression free rates were 40%, 37.50%, and 34.78%, respectively.
Table 2.
Tumor response, RECIST.
The 3-year intrahepatic recurrence free-rates for small, intermediate, and large tumor groups were 55.56%, 51.22%, and 44.26%, respectively. According to the log-rank tests, no significant differences in outcomes were observed among the 3 groups for any event (Fig. 1 and Table 3). However, the 3-year local control rates for the small tumor group was significantly higher (97.85%) than the intermediate (71.99%) or large tumor groups (82.14%; P = .0035) (Fig. 2).
Figure 1.
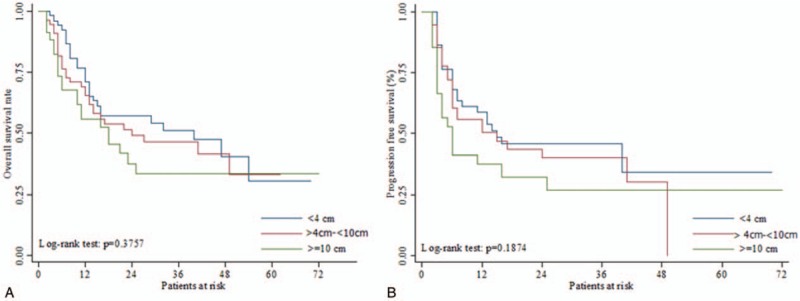
A. Overall survival rate and B. Disease progression free survival rate after SBRT. SBRT = stereotactic body radiation therapy.
Table 3.
Overall survival and control rates at 1, 2, and 3 years for different tumor size after SBRT.
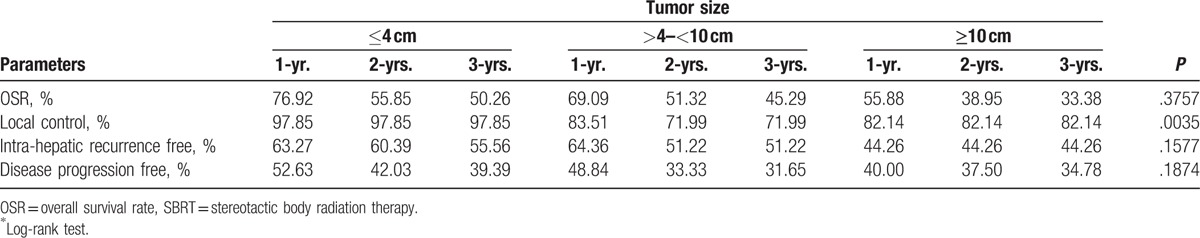
Figure 2.
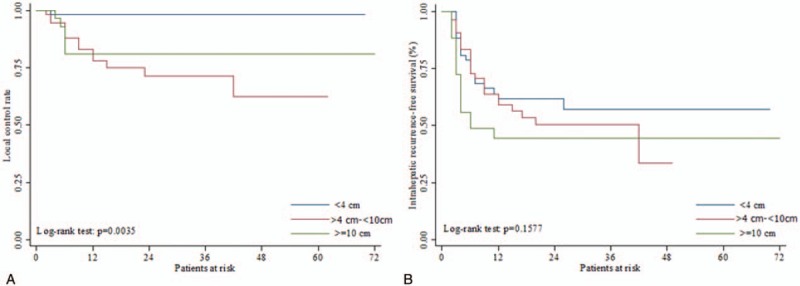
A. Local control rate, B. Intra-hepatic recurrence free survival after SBRT. The 1-year, 2-years and 3-years local control rate for patients with tumor size of ≤4 cm was significantly better among the 3 groups (log-rank test P = .0035). SBRT = stereotactic body radiation therapy.
4.3. Treatment-related toxicity
Acute toxicities are listed in Table 4. The most common acute events were Grade 1–2 fatigue, and the larger the tumor size the more common the fatigue symptoms (48.8% for ≤4 cm, 60% for >4–<10 cm, and 67.65% for ≥10 cm). Other common acute toxicities included Grade 1–2 thrombocytopenia (50% for ≤4 cm, 60% for >4–9 cm, and 55.88% for ≥10 cm) and Grade 1–2 liver enzyme abnormalities which are comparatively similar for all tumor groups. However, larger tumor size tended to have a higher incidence of ≥Grade 3 liver enzyme abnormalities. Radiation-induced dermatitis was found in 3 patients (8.82%) in the large tumor group and 1 patient (1.82%) in the intermediate tumor group since intermediate and large tumors tended to be closer to the abdominal wall and skin. In order to avoid compromising the PTV coverage, chest wall and ribs were not constrained. Nonetheless, all these acute side-effects were transient and patients recovered approximately 1 to 2 weeks later.
Table 4.
Toxicity, CTCAE v 40.
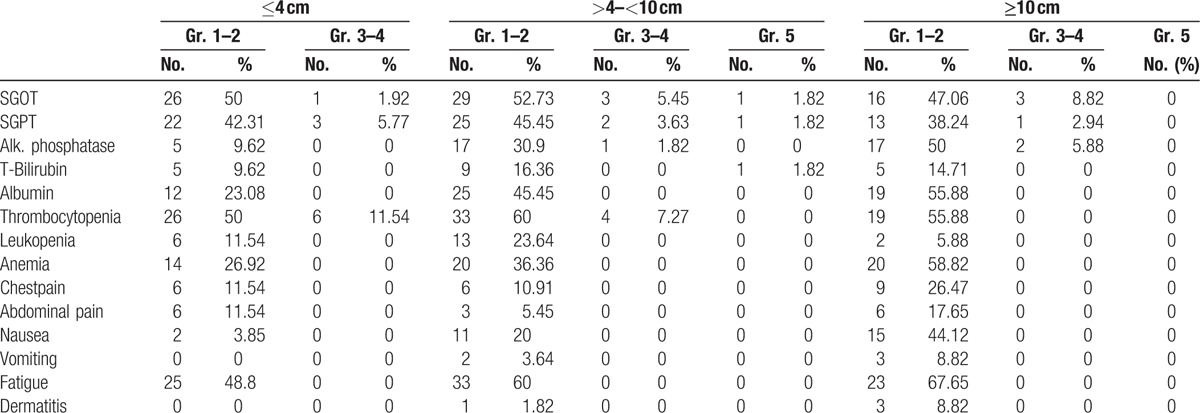
Twelve patients experienced ≥Grade 3 liver function alterations within 3 months of SBRT including 3 patients in the small tumor group, 4 patients in intermediate tumor group, and 5 patients in the large tumor group. All cases were Child-Pugh A. BCLC C was noted in 8 patients, BCLC B in 2 patients, and BCLC A in 1 patient.
Among the causes of liver toxicity, 6 cases were due to disease progression, 5 were non-classic RILD, and 1 was classic RILD. Among the 5 patients with non-classic RILD, 3 were in the intermediate tumor group and 2 belonged to small tumor group, while 1 classic RILD was in the large tumor group. All patients eventually recovered to their previous levels at 1 to 3 months after SBRT. However, a 54-year-old male patient with cT3bN0M0 HCC, HBV liver cirrhosis, Child-Pugh A, and intermediate tumor size went into liver failure after reactivation of HBV titers from failure of prophylactic anti-retroviral medication who died 2 months later.
5. Discussion
SBRT is regarded as a therapeutic option in the management of HCC. Studies of HCC patients treated with SBRT have demonstrated local control rates of 70% to 100%, and it seems that SBRT can increase the local control rate of non-surgical or non-RFA patients, compared with TACE alone. For conventionally fractionated radiation therapy, the probability of tumor control depends on many factors, including tumor size and radiation dose regimen. All else being equal, larger tumors generally require more potent radiation doses compared with smaller tumors to achieve comparable rates of local control. However, because of the underlying liver cirrhosis in most HCC patients, higher doses to larger tumors should be given with caution. An initial review of our data uncovered a potentially confounding association between tumor size and SBRT dose regimen, in which larger tumors tended to have a lesser amount of normal liver volume available and were usually located closer to critical dose limiting organs at risk and, thus, were treated with a less biologically potent SBRT dose regimen (40 Gy/5 fractions).[8] We hypothesized that smaller tumors treated with SBRT would have a better control and survival compared with larger tumors. A previous study by Kwon et al[15] evaluated the long-term effects of SBRT for primarily small HCC (≤100 cm3) ineligible for standard treatment. In their study, 42 HCC patients with tumors ≤100 cm3 were treated with SBRT prescribed at 30 to 39 Gy/3 fractions, resulting in overall 1- and 3-year survival rates of 92.9% and 58.6%, respectively. The local control rates at 1- and 3- years were 72% and 67.5%, respectively. They concluded that patients with smaller tumors had better local control and overall survival rates (<32 cm3 vs ≥32 cm3 (P < .05). A study by Sanuki et al[16] retrospectively analyzed 185 small HCC patients (≤5 cm) treated with SBRT. Two dose levels were prescribed including 40 Gy for Child A and 35 Gy for Child B, in 5 fractions. The 3-year local control and overall survival rates in the 35-Gy and 40-Gy groups were 91% and 89% (log-rank P = .99) and 66% and 72% (P = .54), respectively. Doses of either 35 Gy or 40 Gy in 5 fractions provided equivalent outcomes and were safe. Other studies, including Takeda et al,[17] reported the highest local control rate of >90% for tumor <100 cm3, but it was probably attributed to the fact that 14 of their 16 patients underwent combined TACE prior to SBRT. While Wulf et al[18] reported a local control rate of 100% for a median tumor volume of 114 cm3, the overall survival rate was only 20% at 2 years because of intrahepatic metastasis and progression.
Our study showed a 3-year local control rate and response rate for tumor size ≤4 cm of 97.85% and 96.15% [CR (75.92%) + PR (19.23%)], respectively, which were significantly higher than those from the intermediate and large tumor groups. This result showed that tumor size does have an impact on local control and response rate. However, tumor size did not affect the rate of intrahepatic recurrence-free survival or disease progression-free survival. The overall survival rates at 1-year, 2-years, and 3-years for small tumors (≤4 cm) showed a relatively higher survival rate than those for intermediate and larger sized tumors. However, the trend did not reach statistical significance (P = .3757). Since HCC is a multicentric disease by nature, and since SBRT is a local treatment, intrahepatic-recurrence and disease progression outside the treatment field remain the dominant pattern of relapse following SBRT in our study. The implication is that close regular monitoring of target lesion and metastases is essential, and the combination of SBRT with systemic treatment may potentially increase the overall survival rate and local control (in particular) for larger tumors. Bertino et al,[19] reported that as our knowledge of molecular hepatocarcinogenesis broadened, several molecular targeted agents have been evaluated in clinical trials in advanced HCC. Despite a modest objective response rate, several studies showed encouraging results in terms of prolongation of the time to progression, local control, and overall survival. In addition, we found that patients who achieved initial in-field CR even for larger tumors had a sustained local control throughout the follow-up period, suggesting that initial in-field response and in-field progression are important overall survival indicators. Most CR for smaller tumors (≤4 cm) usually occurred in <3 months after SBRT, while for larger tumors achieving a CR tended to occur at >3 months to 6 months after SBRT.
In terms of toxicity, the present study observed acute toxicities ≥Grade 3 in only 12 patients among the 3 different tumor groups (i.e., 3 in the small tumor group, 4 in the intermediate tumor group, and 5 in the large tumor group). Of the 6 documented cases of RILD in our study, 2 belonged to the small tumor group, 3 to the intermediate tumor group, and 1 to the large tumor group. All cases eventually recovered with the exception of 1 patient who died as a result of radiation-induced liver failure 2 months after treatment; the cause of RILD in this patient was the reactivation of hepatitis B virus rather than tumor size. Based on our results, tumor size was not a factor responsible for severe liver toxicity. However, tumor size did affect Grade 1–2 acute toxicities such as fatigue, nausea, anemia, and thrombocytopenia which were more prominent in large tumor group. Reactivation of hepatitis B virus remains one of the causes of RILD in radiation therapy of liver cancer. Huang et al[7] and Janoray et al[20] reported a RILD incidence rate of 5.5% and 0% or 9%, respectively, using Cyberknife SBRT.
Our study had several limitations including its retrospective nature and the fact that it was performed at a single-institution with a heterogeneous sample size. In addition, bias between groups occurred with respect to the dose regimen. Higher radiation dose was given to smaller tumors and a lower dose regimen was given to intermediate and larger sized tumors. Nevertheless, equivalent overall survival in the 3 groups with different prognosis is beyond the scope of this retrospective study.
6. Conclusions
In conclusion, SBRT provided excellent in-field responses in HCC patients. Those with smaller tumor sizes achieved the best response and local control. Patients who achieved CR for the in-field lesions maintained in-field CR during the follow-up period. Furthermore, this study showed that SBRT was not only a feasible and effective treatment for small tumors (≤4 cm) but could also provide a sustained local control for intermediate (>4–<10 cm) and large sized tumors (≥10 cm). These results suggest that SBRT is a promising, noninvasive modality when HCC is deemed ineligible for surgical resection or ablation therapy. Patterns of failure remain intrahepatic recurrence and disease progression outside the target field, providing a rationale for combining SBRT with regional or systemic therapies, particularly if SBRT is used in healthier, higher performing patients.
Acknowledgments
The authors would like to express their sincere thanks to Hank Ho, Ph.D. for his statistical analyses of the data.
Footnotes
Abbreviations: BCLC= Barcelona clinic liver cancer stage, BED= biological effective dose, CR = complete response, CTV = clinical target volume, ECOG = Eastern Cooperative Oncology Group, GTV = gross tumor volume, HCC = hepatocellular carcinoma, NTD = normalized total dose, PD = progressive disease, PR = partial response, PTV = planning target volume, RFA = radiofrequency ablation, RILD = radiation-induced liver disease, SBRT = stereotactic body radiation therapy, SD = stable, TACE = transarterial chemoembolization.
Authorship: HTK and JQ contributed equally to this work and serve as co-first authors.
Ethics approval and consent to participate: Written informed consent was obtained from all patients before treatment, and the study was approved by the institutional review board of Chi Mei Medical Center.
Funding: This study was self-funding.
The authors declare they have no competing interests.
References
- [1].Ferlay J, Shin H, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [2].Bruix J, Sheramn M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- [3].Toro A, Ardiri A, Mannnino M, et al. Effect of pre-and post-treatment α-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: a retrospective study. BMC Surg 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with 2-f tip microcathether for small hepatocellular carcinomas: Relation ship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007;18:365–76. [DOI] [PubMed] [Google Scholar]
- [5].Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat 2010;9:479–87. [DOI] [PubMed] [Google Scholar]
- [6].Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657–64. [DOI] [PubMed] [Google Scholar]
- [7].Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355–61. [DOI] [PubMed] [Google Scholar]
- [8].Que J, Kuo HT, Lin LC, et al. Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma. BMC Cancer 2016;16:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maluccio M, Covey A, Porat LB, et al. Transcathether arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008;19:862–9. [DOI] [PubMed] [Google Scholar]
- [10].Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Toro A, Bertino G, Arcerito MC, et al. A lethal complication after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Case Rep Surg 2015;2015:873601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim JH, Park JW, Kim TH, et al. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2007;69:813–9. [DOI] [PubMed] [Google Scholar]
- [13].Hoogeman M, Prevost JB, Nuyttens J, et al. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys 2009;74:297–303. [DOI] [PubMed] [Google Scholar]
- [14].Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76(Suppl):S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kwon JH, Baer SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399–404. [DOI] [PubMed] [Google Scholar]
- [17].Takeda A, Takashi M, Kunieda E, et al. Hypofractionated stereotactic radiotherapy with and without intraarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: preliminary results for efficacy and toxicity. Hepatol Res 2007;38:60–9. [DOI] [PubMed] [Google Scholar]
- [18].Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 2006;45:838–47. [DOI] [PubMed] [Google Scholar]
- [19].Bertino G, Di Carlo I, Ardiri A, et al. Systemic therapies in hepatocellular carcinoma: present and future. Future Oncol 2013;9:1533–48. [DOI] [PubMed] [Google Scholar]
- [20].Janoray G, Chapel S, Ruffier-Loubiere A, et al. Robotic stereotactic radiation therapy for tumors of the liver: radiation-induced liver disease, incidence and predictive factors. Cancer Radiother 2014;18:191–7. [DOI] [PubMed] [Google Scholar]


