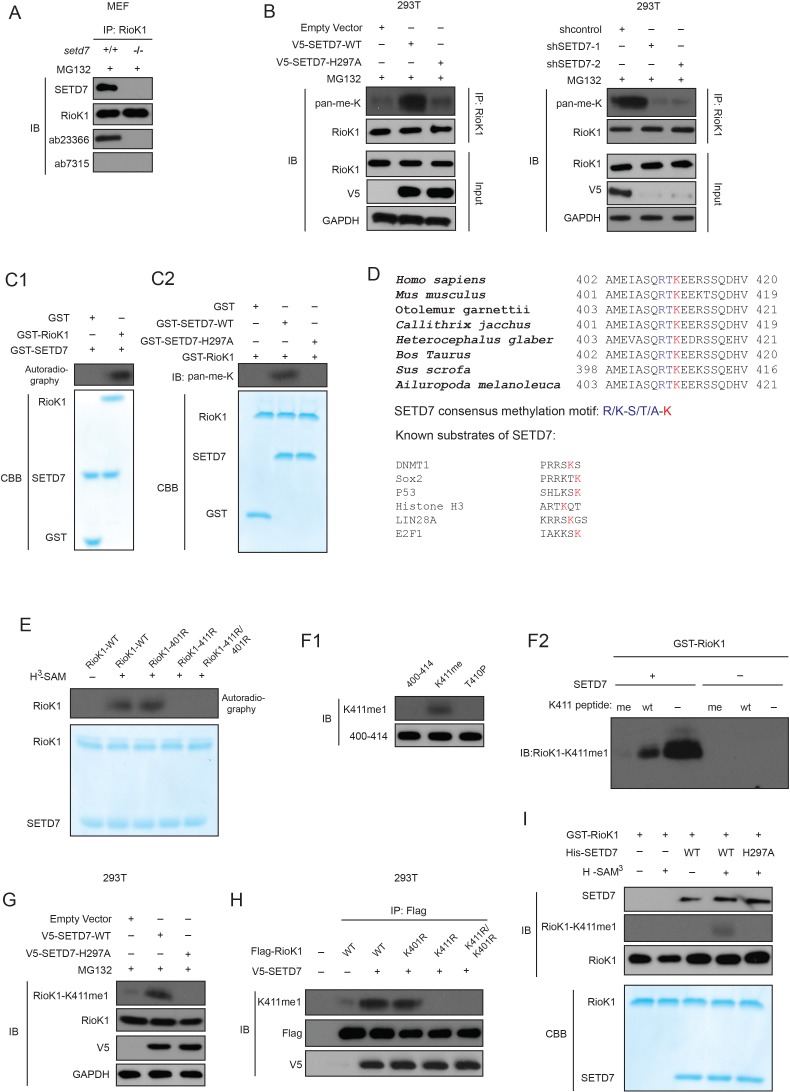
Figure 3. SETD7 monomethylates RIOK1 on lysine 411 in vitro and in vivo.
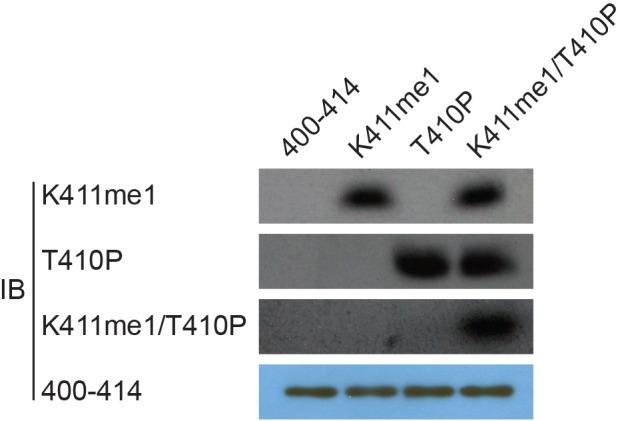
(A) Anti-RIOK1 immunoprecipitates from high-density Setd7+/+ and Setd7−/− MEFs were immunoblotted for RIOK1, SETD7, methyl-lysine (mono-, dimethylated) (ab23366), and dimethyl-lysine (ab7315) antibodies, respectively. (B) SETD7 methylates RIOK1 in vivo. Stably expressed control, SETD7-WT, or SETD7-H297A HEK293T cells were generated. The cell lysates were then precipitated with an anti–RIOK1 antibody and probed with an anti–pan-me-K antibody to detect methylation of RIOK1 (left panel). Stably expressed shcontrol or sh-SETD7 HEK293T cells were generated. The cell lysates were then precipitated with an anti–RIOK1 antibody and probed with an anti–pan-me-K antibody to detect methylation of RIOK1 (right panel). (C1) An in vitro methylation assay was performed with 3H-SAM, recombinant SETD7, and RIOK1. Autoradiography and Coomassie brilliant blue R-250 staining (CBB) were used to show methylation and protein levels, respectively. (C2) In vitro methylation analysis using indicated proteins. An anti–pan-methyl-lysine antibody (pan-me-K) was used to detect the methylation of RIOK1. CBB was used to show the protein levels. (D) Alignment of the consensus amino acid residues adjacent to lysine targeted by SETD7, and identification of a putative SETD7 methylation site in RIOK1 (upper panel). The consensus SETD7 recognition sequence (middle panel). The lysines targeted for methylation by SET7 in known substrates are shown (lower panel). In each case, the methylated lysine is shown in red. (E) In vitro methylation of various purified RIOK1 mutants by SETD7. (F1) An anti-RIOK1-K411me1-specific antibody is characterized. RIOK1 peptide substrates: 400–414 SKAMEIASQRTKEER(Lane 1), K411me SKAMEIASQRTKmeEER(Lane 2), T410p SKAMEIASQRTpKEER(Lane 3), p: phosphorylation; me: methylation. The above peptides were immunoblotted with indicated antibodies. (F2) GST-RIOK1 (1 μg) was methylated in vitro in the presence or absence of recombinant SETD7 (0.5 μg). The reaction was probed with the anti-RIOK1-K411me1 antibody, in the presence of 10 μg/ml competing wild-type (wt) or mono-methylated K411 peptide (me). (G) The effect of ectopically expressed SETD7 and SETD7-H297A on K411me1 of endogenous RIOK1 in HEK293T cells. (H) Methylation analysis of Flag-RIOK1 and mutants by SETD7 in transfected HEK293T cells. (I) In vitro methylation was performed with GST-RIOK1, S-adenosylmethionine (SAM), recombinant His-SETD7, and His-SET7-H297A. Immunoblotting and CBB were used to show methylation and protein levels, respectively.
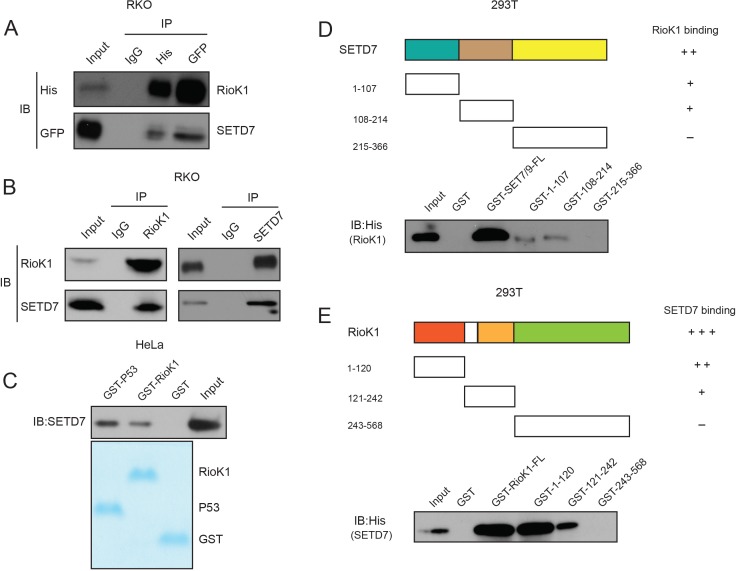
Figure 3—figure supplement 1. SET7/9 directly interacts with RIOK1 in vitro and in vivo.
Figure 3—figure supplement 2. Validation of the antibodies’ specificity.