Fig. 1. Construction of IPR8-SM(PEG)4-R848 conjugate vaccine and biological activity on RAW 264.7 cells.
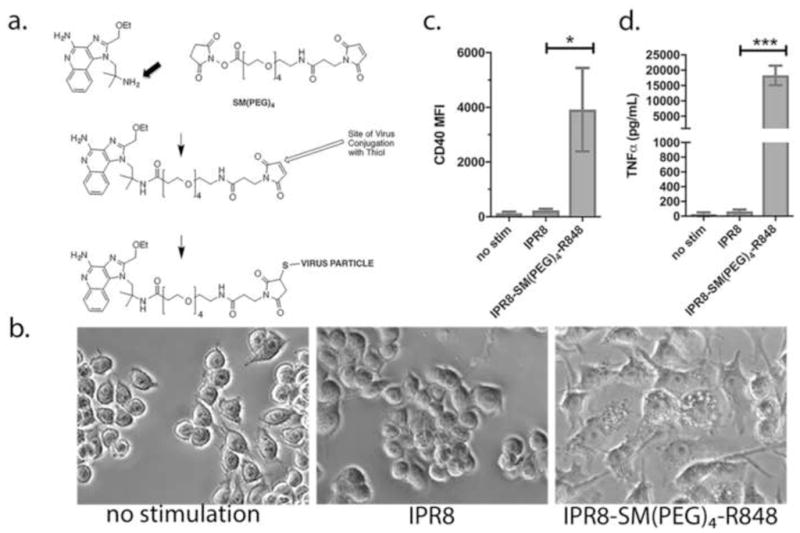
(a) An amine-derivative of R848 (substitution of native OH group with NH2 group is indicated by an arrow) was synthesized to allow for amide bond formation with the reactive NHS-ester end of the amide-sulfhydryl crosslinker, SM(PEG)4. in step 1. Purified PR8 influenza virus was linked via free thiols with the maleimide end of the SM(PEG)4 crosslinker in step 2. To measure biological activity, RAW 264.7 cells were treated with 10 μg/ml IPR8-SM(PEG)4-R848 or unconjugated IPR8 (without the R848 modification). (b) Morphology of cells 24 hrs after addition of IPR8 or IPR8-SM(PEG)4-R848, or no stimulation as a control. As a quantitative measure of activation, CD40 (c) and TNFα (d) production were measured at 24 hrs. Results represent the mean ± sd from 3 experiments. p = 0.01 for CD40 and p = 0.0006 for TNFα, IPR8 vs IPR8-R848-SM(PEG)4 as determined by unpaired Student’s t test.
