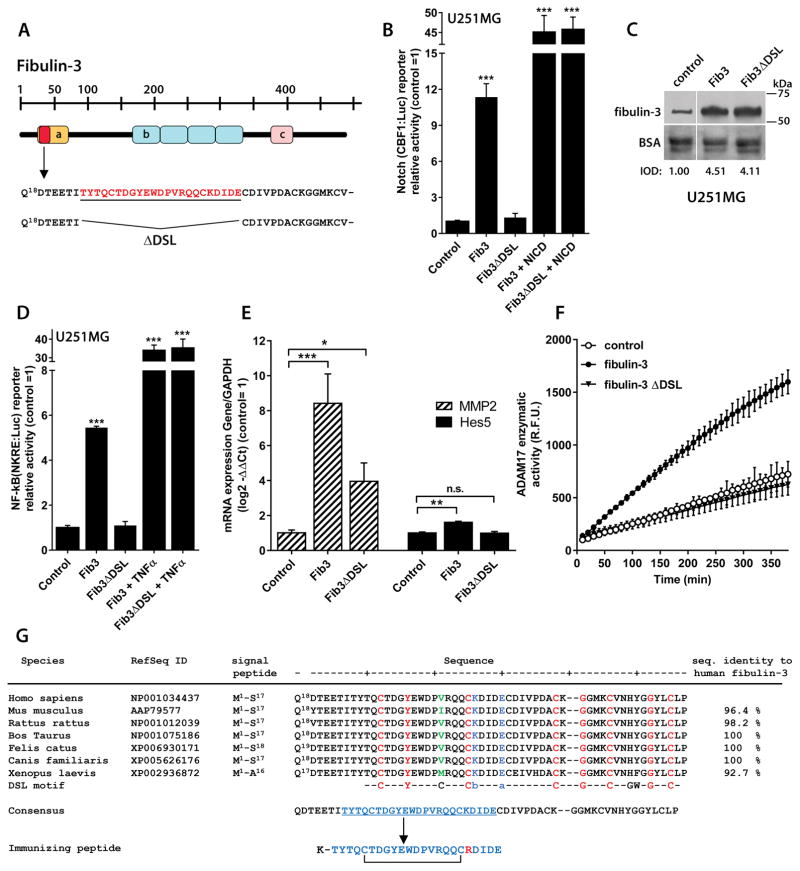
Figure 1. Fibulin-3 signaling depends on a critical N-terminal sequence.
A) Representation of fibulin-3 structure and N-terminal localization of the DSL-like epitope (Thr25-Glu47, underlined) deleted in fibulin-3ΔDSL (a: Ca++-binding EGF-like domain; b: EGF-like repeats; c: fibulin consensus domain). B) The activity of a Notch-dependent luciferase reporter in U251MG cells was increased by fibulin-3 transiently transfected for 24h but not by fibulin-3ΔDSL (control: empty expression vector). None of the fibulin-3 constructs affected Notch reporter activity driven by a constitutive control (NICD). C) Internal transfection controls (10 μg total protein/lane) show that fibulin-3 and fibulin-3ΔDSL were expressed at similar levels in the conditioned medium of U251MG cells; total BSA in the medium was used as loading control (IOD: integrated optical density, relative to the control transfection). D) Transfected fibulin-3, but not fibulin-3ΔDSL, also increased the activity of a NF-κB-dependent reporter in U251MG cells (same experimental design as in (B)). For a positive control of NF-κB-driven reporter activity the cells were treated with TNFα (10 ng/ml, 6h). E) U251MG cells transfected with fibulin-3 cDNA and processed after 24h showed increased mRNA expression of Notch-regulated genes (MMP2 and HES5); transfection of fibulin-3ΔDSL had a much smaller or absent effect. Analyses in (B)–(E) by 1-way ANOVA: *** p< 0.001; ** p<0.01; * p<0.05 for each reporter or gene. F) Overexpression of fibulin-3 in U251MG cells significantly increased the proteolytic activity of ADAM17 in cell lysates (R.F.U.: relative fluorescence units); in contrast, transfection of fibulin-3ΔDSL was undistinguishable from control cells (all cells processed 24h post-transfection). G) Alignment of the N-terminal sequence of fibulin-3 against the canonical DSL-motif of Notch ligands; this sequence is very well conserved across species. Amino acids in red indicate conservation of DSL between fibulin-3 and Notch ligands and those in green show a major divergence from the consensus DSL motif (b: basic amino acid; a: acidic amino acid). The peptide chosen for immunization corresponds to Thr25-Glu47 of human fibulin-3 but has Lys43 replaced with Arg as indicated in the Methods section.