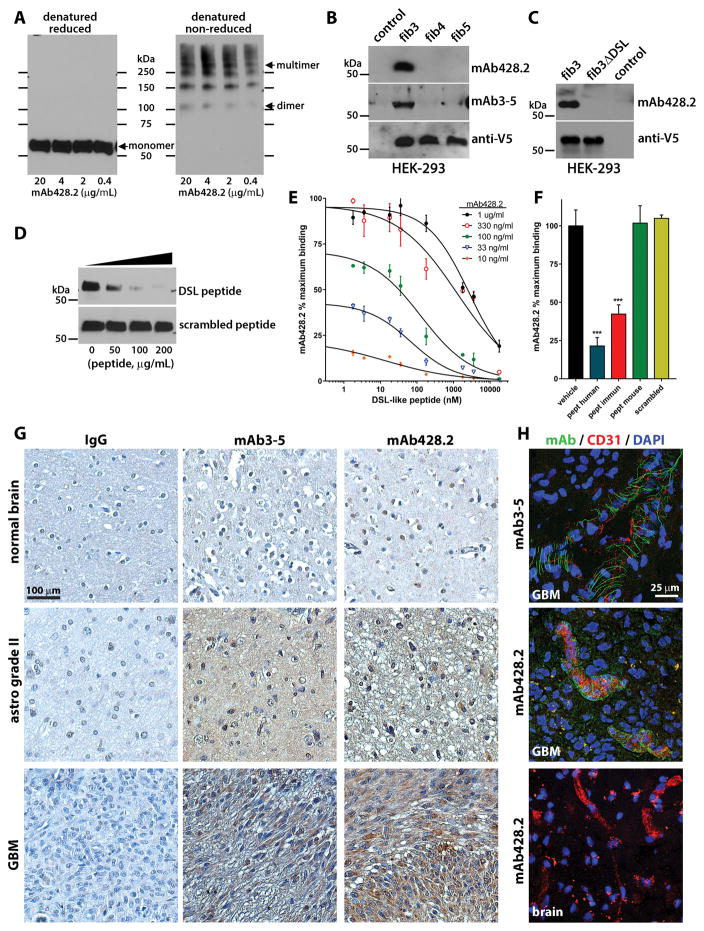
Figure 2. Characterization of anti-fibulin-3 mAb428.2.
A) Western blots showing detection of purified fibulin-3 by mAb428.2 in reduced and non-reduced conditions (200 ng protein/lane). The arrows indicate the position of the 55-kDa monomer, 110-kDa dimer, and high-Mw multimer (fibrillar) forms of fibulin-3. B) Western blots of serum-free conditioned medium from HEK293 cells (10 μg total protein/lane) expressing V5-tagged fibulins -3, -4, or -5. Blots were probed with an antibody against the V5 epitope or with the anti-fibulin-3 antibodies mAb428.2 and mAb3-5. The control lane contains medium from untransfected cells. C) Western blots of serum-free conditioned medium from HEK293 cells expressing V5-tagged full-length fibulin-3 (fib3) and fibulin-3ΔDSL that lacks the target epitope of mAb428.2. D) Detection of purified fibulin-3 (200 ng/lane) by mAb428.2 was inhibited by the DSL-like peptide from fibulin-3 (Thr25-Glu47) but not by a scrambled version of this peptide. E) Microtiter plates were coated with BSA-conjugated DSL-like peptide (1,000 ng/ml) and different concentrations of mAb428.2 were used to detect the epitope by indirect-ELISA. mAb428.2 binding to BSA-conjugated peptide was displaced by incubation with free DSL-like peptide following a simple competitive model (analyzed in Suppl. Figure S2A). F) Microtiter plates were coated with purified fibulin-3 (200 ng/ml) and mAb428.2 (1 μg/ml) was used to detect the protein by indirect-ELISA. Binding of the antibody was inhibited by the DSL-like peptide from human fibulin-3 (pept human) and the modified peptide used for immunization (pept immun); *** p<0.001, 1-way ANOVA. However, the DSL-like peptide from mouse fibulin-3 (pept mouse, containing Ile38 instead of Val38) was unable to displace mAb428.2 binding. All peptides were tested at a maximum concentration of 50 μg/ml (18 μM). G) Immunohistochemistry of paraffin-processed tissue sections using mAb428.2 or mAb3-5 showed similar detection of fibulin-3, which increased with tumor grade. The sparse nuclear staining observed with both antibodies is non-specific. Control staining was performed with non-immune mouse IgG. H) Immunohistochemistry of frozen GBM sections using mAb428.2 or mAb3-5 showed a characteristic perivascular fibrillar pattern previously described (28). mAb428.2 did not detect perivascular fibulin-3 in blood vessels from normal brain adjacent to the tumor (brain), as expected. Vessels were stained with an antibody against endothelial CD31.