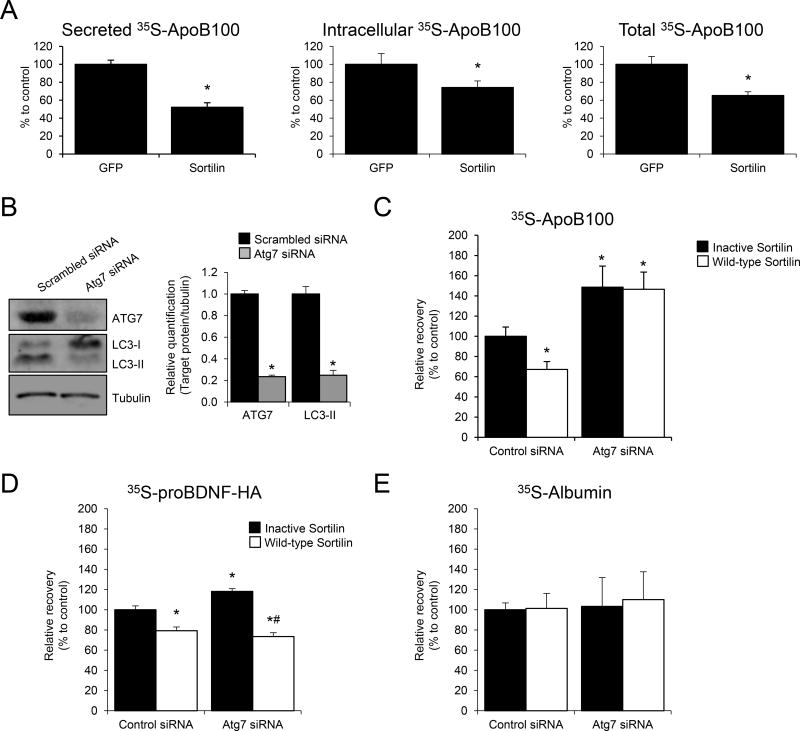
Figure 1. Sortilin overexpression increases apoB100 degradation in rat hepatic cells in an autophagy-dependent manner.
(A) McA rat hepatic cells stably expressing GFP or sortilin were cultured in normal growth medium. When cells reached sub-confluence (80%–90%), they were washed with PBS, and amino acid-starved for 1 h before being subjected to steady state metabolic labeling using a mixture of [35S]-methionine and cysteine. After 4 h, cells were harvested and apoB100 was immunoprecipitated, separated by SDS-PAGE, and bands quantified using a phosphorimager. The graphical data are displayed as the relative differences in secreted apoB100 (left panel), intracellular apoB100 (middle panel), and total (cell+medium), right panel apoB100 between the cells transfected with GFP (set to 100%) vs. sortilin. (B–E) McA hepatic cells stably expressing human apoB100 were transfected with Atg7 siRNA or with scrambled siRNA for 24 h. Then, cells were transfected with either a plasmid containing the full-length myc-tagged human sortilin (Wild-type Sortilin, pcDNA3.1-hSORT1-myc) or a dominant negative sortilin (“Inactive Sortilin”; pcDNA3.1-hPro-Sortilin) generated by mutation of the furin recognition site, which prevents sortilin binding to apoB100. (B) Western blot for ATG7, LC3-I and LC3-II levels 48 h after siRNA incubation. Tubulin was used as internal control. (C) Pulse-chase results showing the recoveries of apoB100 at the end versus beginning of the chase period, with “Inactive Sortilin, Control siRNA” set to 100%. (D) Relative recovery of proBDNF-HA from McA hepatic cells transfected with Atg7 siRNA or with scrambled siRNA for 24 h. Prior to the pulse-chase study, an expression plasmid for proBDNF-HA was co-transfected with an expression plasmid for either wild-type or inactive sortilin for 24 h. (E) Relative recovery of albumin. Numerical data represent the means ± SEM. *, p<0.05 versus Control siRNA; #, p<0.05 versus Atg7 siRNA using two-tailed Student’s t-test. The experiments were performed in triplicate.