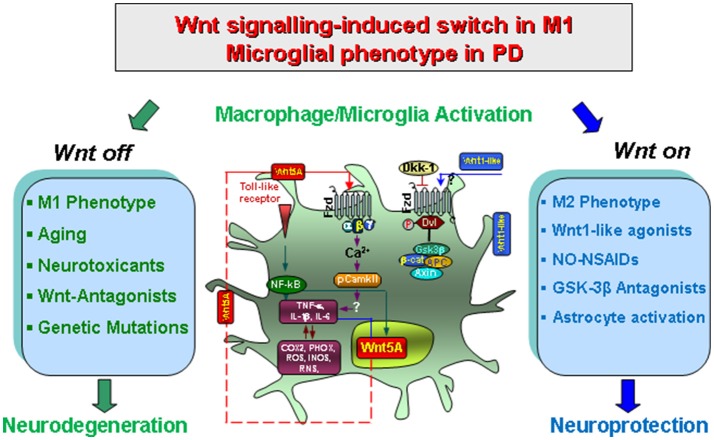
Figure 2.
Wnt/β-catenin signaling-induced switch in proinflammatory microglial M1 phenotype in concert with gene-environment interactions in PD. Upon activation by neurotoxins, endotoxins or brain injury and aging, macrophage/microglia produce a panel of pro-inflammatory cytokines (TNF-α and IL-1β) and chemokines (CCL3, CXCl10 and CXCL11), of which Wnt5a constitutes one part of a self-perpetrating cycle, via autocrine Wnt5A/CamKII activation and paracrine stimulation of Th-1- cytokines, iNOS and COX2 (Pereira et al., 2009; Neumann et al., 2010; Halleskog et al., 2012). Up-regulation of microglial PHOX-derived ROS, iNOS-derived NO, and GSK-3β, a known regulator of NF-kB-dependent gene transcription, further exacerbate microglia reaction (Beurel et al., 2010; L'Episcopo et al., 2012, 2013). In addition, astrocyte-derived growth/neurotrophic and anti-oxidant factors including Wnt1, can mitigate the inflammatory milieu and favor a progressive neurorescue program for mDA neurons (Marchetti et al., 2013). However, an exaggerated M1 microglial pro-inflammatory status as observed with age, MPTP exposure, and synergy with different gene and environmental risk factors can impair astrocyte anti-inflammatory and neuroprotective functions also via inhibition of Wnt1 expression and downregulation of anti-oxidant/anti-inflammatory cytoprotective proteins in astrocytes (L'Episcopo et al., 2013). Modified from Marchetti and Pluchino (2013), with permission.