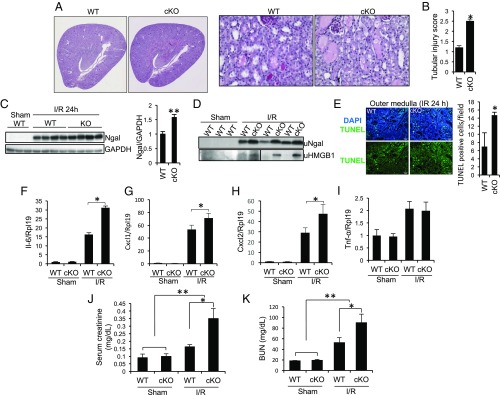
Fig. 1.
Tubule-specific ablation of Rgmb aggravates IRI. Male WT and Rgmb cKO mice at 2 mo of age underwent 40 min of bilateral renal pedicle clipping. Mice were killed 24 h after reperfusion. (A) Periodic acid-Schiff (PAS) staining of kidney sections from WT and Rgmb cKO mice after IR. Representative photographs at different magnifications show more brush border loss, tubular dilation, tubular cell depletion, and cast formation in Rgmb cKO mice than in WT mice. (B) Quantitative assessment of tubular injury is presented. (C) Expression of Ngal in the kidneys of WT and Rgmb cKO after IR. Kidney lysates from sham-operated kidneys and injured kidneys were subjected to Western blotting for Ngal (Left), and quantified by densitometry (Right). (D) Excreted Ngal and HMGB1 in urine (uNgal and uHMGB1). Spot urinary samples were collected from sham-operated mice and mice with IR when they were killed. The same volume of samples was used for Western blotting. (E) TUNEL-positive tubular cells in outer medulla. Paraffin kidney sections from WT and Rgmb cKO mice were used for TUNEL staining (Left). Nuclear localization of TUNEL signal was demonstrated by cyan-colored nuclei after merging with DAPI. Positive tubular epithelial cells in each field were counted (Right). (F–I) Expression of inflammatory factors in the kidney. Kidney lysates from sham-operated kidneys and injured kidneys were used for real-time PCR analysis for Il-6, Cxcl1, Cxcl2, and Tnf-α. (J and K) Serum creatinine and BUN levels in sham-operated mice and in mice with IR. Rpl19 was used as the internal control for real-time PCR. GAPDH was used as the loading control for Western blotting. n = 6–8 for B and F–K; n = 3 for E. *P < 0.05; **P < 0.01.