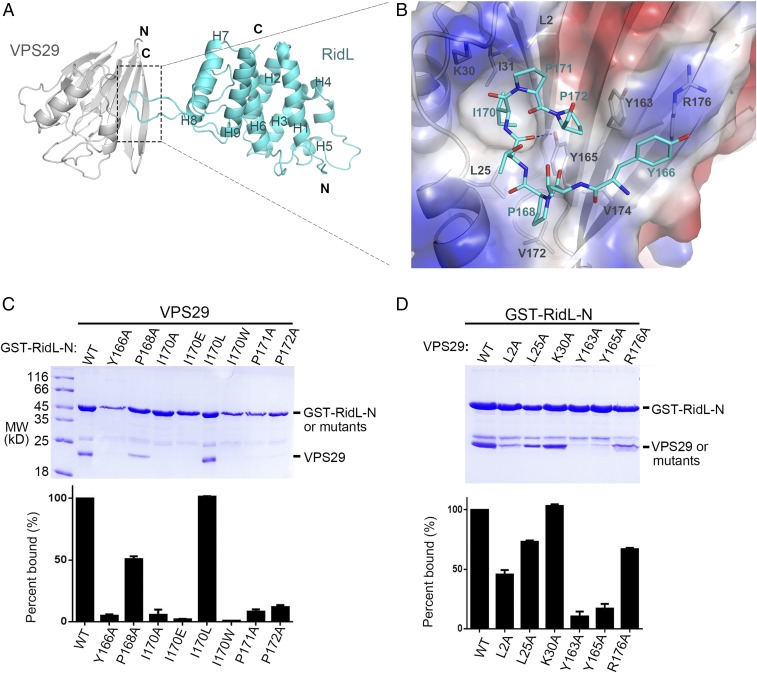
Fig. 2.
Structural mechanism of VPS29 recognition by RidL-N. (A) Ribbon diagram of the VPS29/RidL complex (VPS29, gray; RidL, cyan). N and C terminus of proteins are labeled. The secondary structure elements of RidL are labeled. (B) VPS29–RidL interactions in detail. Critical VPS29 and RidL residues are shown in stick modes. A partially transparent electrostatic surface potential map is presented for VPS29. Blue dash represents hydrogen bond between RidL and VPS29. (C and D) Coomassie blue stained SDS/PAGE gels of bound proteins are shown. Results are representative of three independent experiments. Amount of VPS29 retained was expressed relative to the amount of GST–RidL in the bound sample and then normalized to the amount of wild-type protein. All values are presented as mean ± SD, derived from three independent experiments. In C, immobilized GST–RidL-N or its mutants (Y166A, P168A, I170A, I170E, I70L, I170W, P171A, and P172A) was used to pull down VPS29. In D, immobilized GST–RidL-N was used to pull down VPS29 WT or mutants (L2A, L25A, K30A, Y163A, Y165A, and R176A).