Fig. 3.
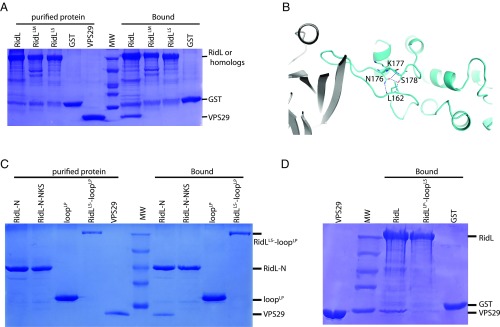
The sequence and conformation of the RidL loop are essential for the binding to VPS29. (A) GST–RidL from related Legionella species pull-down of purified VPS29. Shown are Coomassie blue stained SDS/PAGE gels of purified proteins used (Left) and bound samples (Right). (B) Detailed VPS29–RidL interactions highlight RidL residues that are distant from the binding sites of VPS29, but critical for the conformation of the binding loop. Residues L162, N176, K177, and S178 are shown in stick representation. Hydrogen bonds are denoted with blue dash lines. (C) GST–RidL-N, RidL-N-NKS, loopLP, RidLLS-loopLP pull-down of purified VPS29. Shown are Coomassie blue stained SDS/PAGE gels of purified proteins used (Left) and bound samples (Right). (D) GST–RidL, RidLLP-loopLS, and GST pull-down of purified VPS29. Shown are Coomassie blue stained SDS/PAGE gels of purified VPS29 protein (Left) and bound samples (Right).