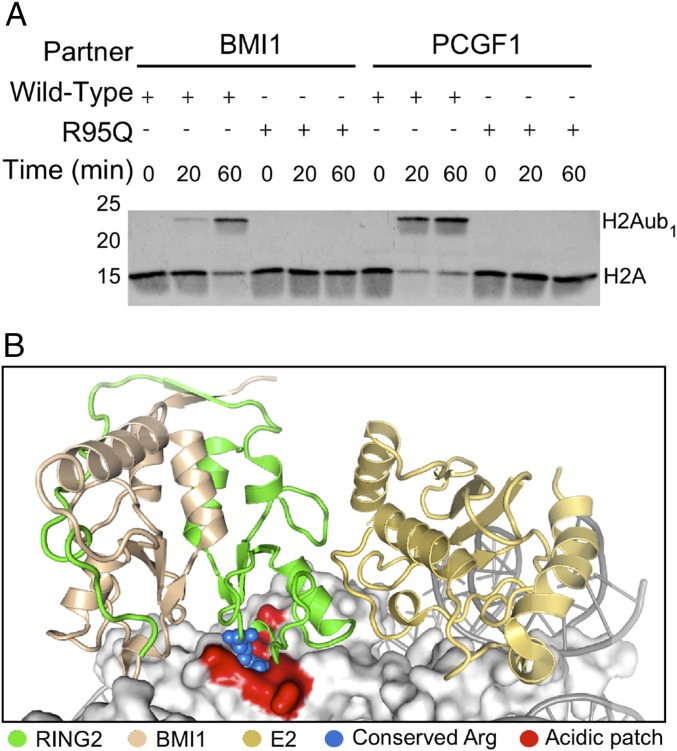
Fig. 2.
RING 1 p.R95Q causes a defect in ubiquitylation of histone H2A specifically on nucleosomes. (A) In vitro nucleosome ubiquitylation assay of wild-type RING1 or RING1 p.R95Q heterodimers with BMI1 or PCGF1. All proteins and nucleosomes were expressed and purified, and protein concentrations in samples were matched using UV absorbance at 280 nm. Histone H2A was detected by immunoblotting. The RING1 p.R95Q mutant is deficient at ubiquitylating H2A in nucleosomes. (B) Structure of the RING2/BMI1 complex, showing BMI1 (tan), RING2 (green), and E2 (gold) bound to a nucleosome (gray) (PDB ID code 4R8P). The structure highlights the contact between the conserved positive arginine residue (blue spheres) and the nucleosome acidic patch (shown in red).