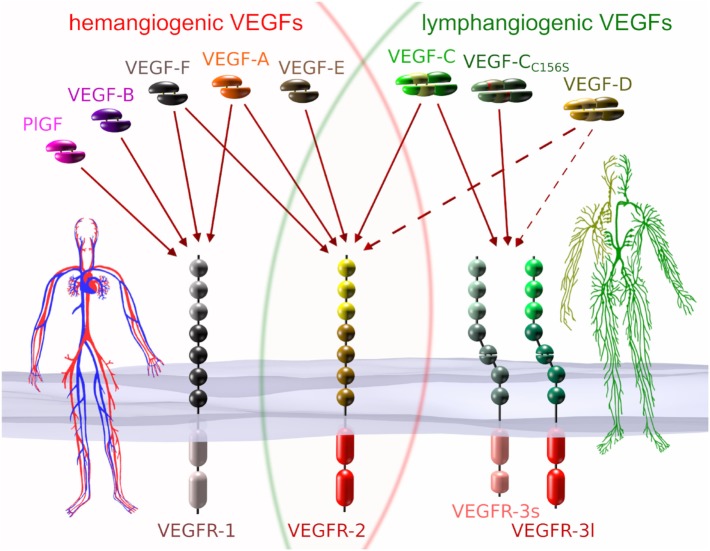
Figure 1.
VEGFs and VEGF receptors (VEGFRs). Each of the five mammalian VEGFs [PlGF, VEGF-A to -D], the viral VEGF-E, and the snake venom VEGF-F interacts specifically with a certain subset of the three VEGFRs. VEGF-CC156S is an engineered vascular endothelial growth factor C (VEGF-C) variant that interacts predominantly with VEGF receptor 3 (VEGFR-3) (Joukov et al., 1998). VEGFs that interact with all three receptors do not naturally exist, but have been engineered (Jeltsch et al., 2006). VEGF receptor 1 (VEGFR-1) and VEGF receptor 2 (VEGFR-2) are expressed on blood vascular endothelial cells (BECs), while VEGFR-2 and VEGFR-3 are expressed on lymphatic endothelial cells. VEGFR-3 is the primary mitogenic receptor for lymphatic endothelium, while VEGFR-2 is the primary mitogenic receptor for blood vascular endothelium. Exclusive to higher primates is the appearance of a short splice isoform of VEGFR-3 (VEGFR-3s) (Pajusola et al., 1993; Borg et al., 1995; Hughes, 2001). Signaling pathways activated by VEGFR-3s are partially distinct from those activated by the long splice isoform (VEGFR-3l), since it lacks some of the phosphorylation sites required for mediator docking (e.g., for Shc-Grb2) (Fournier et al., 1995; Dixelius et al., 2003). The dotted arrows from VEGF-D indicate heterogeneous binding patterns. While mature human VEGF-D can activate VEGFR-2, this seems not to be the case for mouse VEGF-D (Baldwin et al., 2001), and consequently, VEGF-D function could have diverged since the evolutionary divide some 60–65 million years ago (O’Leary et al., 2013). Additionally, human VEGF-D can selectively lose its affinity for VEGFR-3 after proteolytic processing (Leppanen et al., 2011).