Abstract
Objective
Lauric acid (C12) is a medium-chain fatty acid that inhibits growth and production of the greenhouse gas methane by rumen methanogens such as Methanobrevibacter ruminantium. To understand the inhibitory mechanism of C12, a transcriptome analysis was performed in M. ruminantium strain M1 (DSM 1093) using RNA-Seq.
Results
Pure cell cultures in the exponential growth phase were treated with 0.4 mg/ml C12, dissolved in dimethyl sulfoxide (DMSO), for 1 h and transcriptomic changes were compared to DMSO-only treated cells (final DMSO concentration 0.2%). Exposure to C12 resulted in differential expression of 163 of the 2280 genes in the M1 genome (maximum log2-fold change 6.6). Remarkably, C12 hardly affected the expression of genes involved in methanogenesis. Instead, most affected genes encode cell-surface associated proteins (adhesion-like proteins, membrane-associated transporters and hydrogenases), and proteins involved in detoxification or DNA-repair processes. Enrichment analysis on the genes regulated in the C12-treated group showed a significant enrichment for categories ‘cell surface’ and ‘mobile elements’ (activated by C12), and for the categories ‘regulation’ and ‘protein fate’ (represssed). These results are useful to generate and test specific hypotheses on the mechanism how C12 affects rumen methanogens.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3242-8) contains supplementary material, which is available to authorized users.
Keywords: Methanobrevibacter ruminantium, Methanogenesis, Fatty acid, Rumen, Gene expression, Lauric acid
Introduction
Ruminal methane-producing archaea acquire attention because ruminant livestock is estimated as the most important source of anthropogenic emission of the greenhouse gas methane [1]. Among the most-promising anti-methanogenic compounds are two medium chain fatty acids (MCFA), lauric acid (C12) and myristic acid (C14), which were shown to inhibit methanogenesis in vivo when supplemented to the diet of ruminants [2–4], in vitro in rumen fluid [5] and in methanogenic cultures [6]. MCFA cause leakage of K+ ions and decrease survival of Methanobrevibacter ruminantium, a dominant methanogen species in the rumen [6, 7]. Further, MCFA killed some, but not all methanogen cells, which implies that the cells may be capable to react to fatty acid-caused stress. In search of the mode of action, we investigated the transcriptional response of M. ruminantium to exposure of C12 in culture.
Main text
Methods
Experimental design
Methanobrevibacter ruminantium (strain M1, DSM 1093; ‘Deutsche Sammlung von Mikroorganismen und Zellkulturen’ (DSMZ), Braunschweig, Germany) was cultivated anaerobically in 50 ml of modified Methanobacterium medium (DSMZ No. 1523) in 116 ml bottles under a CO2/H2 (0.2:0.8) atmosphere at 150 kPa and at 39 °C in an incubation shaker as described previously [6]. Growth of the cultures was monitored by recording optical density at 600 nm and by methane (CH4) formation after 24, 48, 60 and 61 h. The culture was inoculated with 5 ml of an exponentially growing pre-culture (OD600 ~ 0.64) to 45 ml of medium. Cell survival was detected with the LIVE/DEAD BacLight Bacterial Viability Kit for microscopy and quantitative assays (Kit L7012; Invitrogen GmbH, Darmstadt, Germany) [6]. Lauric acid (≥ 97% purity) was obtained from Sigma-Aldrich (Buchs, Switzerland), and a stock solution with 200 mg/ml was prepared by dissolving the C12 in sterile dimethyl sulfoxide (DMSO) (Sigma-Aldrich), a commonly used solvent for water-insoluble substances [8]. After 60 h of incubation, when cells reached the exponential phase, three bottles were supplemented with 0.1 ml of the C12 stock solution to reach a final concentration of 0.4 mg C12/ml (treatment group), three bottles were supplemented with 0.1 ml of DMSO (final concentration: 0.2%) (control group), and three bottles received no supplement (blank group). The concentration of C12 and the exposure time of 1 h chosen were in a range where most cells remained alive and where CH4 formation was clearly but not completely inhibited. It was verified that, at 61 h of incubation, CH4 formation rates and proportion of living cells did not differ between DMSO-exposed control cultures (measured: 0.71 ± 0.03 µmol/ml × h and 97 ± 0.3%, respectively) and untreated blank cultures (0.74 ± 0.04 µmol/ml × h and 99 ± 1.2%). At 61 h, i.e. after 1 h of exposure to C12, CH4 formation rates in the hour after exposure were suppressed by 40 ± 6% compared to the control cultures (P < 0.05), and cell viability was reduced down to 71 ± 1.8% when compared to the control cultures (P < 0.05). At this time point, three samples per group (each 50 ml of culture) were anaerobically collected at 4 °C after centrifugation at 5000×g for 6 min. Cell pellets were immediately frozen in liquid nitrogen and stored at − 80 °C until RNA extraction.
RNA isolation
Total RNA was isolated from the frozen cell pellets by using TRIzol® Reagent (ThermoFisher, Waltham, MS, USA), according to the manufacturer’s protocol. In order to remove genomic DNA from total RNA samples, a DNA digestion was performed with the RNase-Free DNase Set (Qiagen, Hilden, Germany) following manufacturer’s instructions. Quantity and quality of extracted RNA were determined by a Qubit® 1.0 fluorometer with a Qubit RNA BR (Broad Range) assay kit (Invitrogen, Carlsbad, CA, USA) and by an Agilent 2200 TapeStation with the Agilent RNA ScreenTape assay (Agilent Technologies, Santa Clara, CA, USA), respectively. Nine purified total RNA samples with a yield of at least 5 µg and RNA integrity numbers (RIN) in a range of 5.6–7.6 were used for sequencing. These included three replicates per group: three DMSO-dissolved C12-treated samples (T1, T2 and T3), three samples with DMSO supplementation alone (control samples C1, C2, C3) and three samples without supplement (blank samples B1, B2, B3).
Ribosomal RNA depletion
The Ribo-Zero™ rRNA removal kit (Bacteria) (http://www.illumina.com/products/ribo-zero-rrna-removal-bacteria.html, Epicentre, San Diego, USA) was applied to deplete rRNA from the M. ruminantium total RNA samples (5 µg) by following the Illumina user guide for the Ribo-Zero Magnetic kits (Part#15065382 Rev. A, November 2014). The rRNA-depleted samples were purified with AMPure RNAClean XP Beads (Beckman-Coulter Genomics, Nyon, Switzerland) as recommended in the Illumina protocol mentioned above.
Next generation sequencing
Enriched RNA samples were used to produce library constructs by following the Illumina TruSeq® Stranded total RNA protocol (Part#15031048 Rev. C, September 2012) with the Illumina TruSeq Stranded total RNA Sample Preparation Kit. Libraries were quantified and quality checked using qPCR with Illumina adapter specific primers (Roche LightCycler® system, Roche Diagnostics, Basel, Switzerland) and by the Agilent Technologies 2100 Bioanalyzer with DNA-specific chips, respectively. Diluted indexed libraries (10 nM) were pooled, used for cluster generation (Illumina TruSeq SR Cluster Kit v4-cBot-HS reagents) and further sequenced (Illumina TruSeq SBS Kit v4-HS reagents) on the Illumina HiSeq 2500 instrument in the high output mode according to the manufacturer’s recommendations. Illumina single read approach (1 × 125 bp) was used to generate raw sequencing reads with a depth of approximately 20–30 million reads per sample.
RNA-sequencing data analysis
Data analyses were performed as described by Tanner et al. [9]. Shortly, reads (125 bp) were mapped against the genome of M. ruminantium M1 using the CLC Genomics Workbench 6.5.1 (CLC, Aarhus, Denmark). Statistical analysis was performed using Bioconductor EdgeR software package in R. A false discovery rate (FDR) value < 0.05 was used as cutoff for significance of differentially expressed genes and log2 fold change > 1 and < −1 was used as cutoff for differential transcription of genes higher (positive log2-fold change values) or lower (negative log2-fold change values) expressed in cultures [10]. To test for significant enrichment in each category listed in Table 1, a two-tailed Fisher test was performed at http://www.langsrud.com/fisher.htm.
Table 1.
Number of genes significantly differential expressed within functional categories
| Category | Gene count | Treatment vs. control | Control vs. blank | Treatment vs. blank | |||
|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | ||
| Amino acid metabolism | 94 | 2b | 3 | 0 | 4 | 1 | 2 |
| Cell cycle | 29 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cell envelope | 189 | 28a | 0b | 2 | 4 | 2 | 3 |
| Cellular processes | 14 | 3 | 1 | 1 | 0 | 2a | 0 |
| Central carbon metabolism | 61 | 2 | 1 | 0 | 1 | 2 | 0 |
| Energy metabolism | 141 | 9 | 9a | 6 | 3 | 6 | 0 |
| Lipid metabolism | 21 | 0 | 0 | 1 | 0 | 0 | 3a |
| Mobile elements | 87 | 37a | 0 | 0 | 37a | 0 | 0 |
| Nitrogen metabolism | 14 | 0 | 1 | 1 | 0 | 1 | 0 |
| Nucleic acid metabolism | 60 | 2 | 1 | 0 | 0 | 0 | 0 |
| Protein fate | 51 | 0b | 2 | 1 | 0 | 1 | 0 |
| Protein synthesis | 169 | 7 | 1 | 0b | 9 | 0b | 0 |
| Purines and pyrimidines | 47 | 2 | 0 | 0 | 0 | 0 | 0 |
| Regulation | 68 | 0b | 5a | 5a | 0 | 2 | 0 |
| Secondary metabolites | 12 | 4 | 0 | 0 | 0 | 0 | 0 |
| Transcription | 26 | 1 | 0 | 0 | 0 | 0 | 0 |
| Transporters | 97 | 11 | 1 | 7a | 3 | 7a | 1 |
| Unknown function | 183 | 10 | 8 | 4 | 2b | 3 | 0 |
| Vitamins and cofactors | 142 | 8 | 3 | 2 | 4 | 5 | 1 |
| Totalc | 1505 | 127 | 36 | 30 | 67 | 32 | 10 |
aSignificant functional enrichment in a Fisher exact test (p < 0.05)
bSignificant functional underrepresentation in a Fisher exact test (p < 0.05)
cNon-conserved hypothetical genes and RNAs are omitted in the classification [11]. Treatment: with DMSO-dissolved C12, control: with DMSO alone, blank: without C12 and DMSO
Results and discussion
The Ribo-Zero™ rRNA Removal Kit can be used to efficiently remove the rRNA fraction from total RNA samples isolated from the archaeon M. ruminantium M1. The Epicentre probes (directed to bind rRNA from a broad spectrum of bacteria species) reduced the rRNAs in all samples tested, which resulted in 40–85% of non-rRNA sequencing reads in the samples (Fig. 1). More than 10 million mRNA sequencing reads per sample were mapped to the genome of M. ruminantium M1 (Fig. 1), which is a sufficient coverage for transcriptome analyses [11].
Fig. 1.
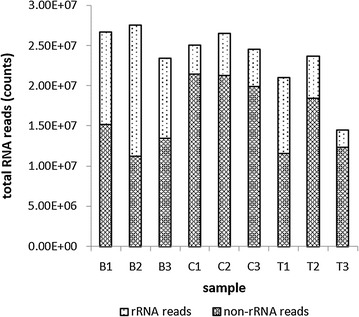
Ribosomal RNA depletion and reads enrichment in RNA extracted from M. ruminantium M1. B: blank (without C12 and dimethyl sulfoxide, DMSO), C: control (with DMSO alone), T: treatment (with DMSO-dissolved C12). Note that the y-axis is non-linear
First, we compared the untreated cultures to the control cultures treated with DMSO. DMSO affected the expression of 97 out of 2280 genes in the M1 genome (Additional file 1). DMSO induced changes in gene expression of cell surface-related proteins, cell membrane-associated transporters and intracellular proteins; the latter maybe related to the observation that DMSO penetrates cell membranes [8]. DMSO-regulated genes included genes encoding proteins related to the cell envelope, mainly adhesion-like proteins (six genes; four down-regulated, two up-regulated). Others were classified as mobile genetic elements (38 genes including hypothetical genes; all down-regulated), and genes involved in energy metabolism, mainly hydrogen metabolism [nine genes, six up-regulated (frhA/B1/D/G, mtrA2, DsbD), three down-regulated (hypA/B, adh3)]. Genes involved in metabolism of vitamins and cofactors (six genes; four down-regulated, two up-regulated) as well as of amino acids (four genes, all down-regulated) were regulated. Moreover, cation transporters (five genes; four of five up-regulated), amino acid transporters (two genes; down-regulated), and other transporters (three genes, up-regulated) showed differential expression when untreated cultures were compared to DMSO-supplemented cultures. Overall, the set of genes regulated in the DMSO control group compared to the blank group was enriched for genes assigned to categories: ‘Mobile elements’, ‘Transporters’, and ‘Regulation’, whereas genes assigned to ‘protein synthesis’ and genes of unknown function were significantly underrepresented (Table 1).
The comparison between the C12 + DMSO-treated and the untreated cultures revealed 42 genes differentially regulated (Additional file 2), 26 of these also found in the DMSO-treated versus untreated comparison (Additional file 3).
Thereafter the transcriptome of the C12 + DMSO-treated and DMSO-treated cultures were compared to identify the mechanisms how MCFA affect methanogenesis. A total of 147 genes, 6.4% of all 2280 genes, were differentially regulated (Table 2).
Table 2.
Significant changes of gene expression in M. ruminantium M1 cultures exposed to C12
| Category and subcategory | ORF | Gene name | Annotated function | log2-fold change | log2 counts per 106 reads |
|---|---|---|---|---|---|
| Amino acid metabolism | |||||
| Lysine | mru_0152 | lysA | Diaminopimelate decarboxylase LysA | − 1.02 | 7.66 |
| mru_0153 | dapF | Diaminopimelate epimerase DapF | − 1.00 | 7.01 | |
| Histidine | mru_0182 | hisH | Imidazole glycerol phosphate synthase glutamine amidotransferase subunit HisH | − 1.07 | 6.27 |
| Serine | mru_0678 | serA | Phosphoglycerate dehydrogenase SerA | 1.03 | 9.59 |
| Tryptophan | mru_2159 | trpB2 | Tryptophan synthase beta subunit TrpB2 | 1.00 | 11.31 |
| Cell cycle | |||||
| Cell division | mru_2160 | minD | Cell division ATPase MinD | 1.08 | 5.46 |
| Cell envelope | |||||
| Cell surface proteins | mru_1500 | mru_1500 | Adhesin-like protein | 1.00 | 8.58 |
| mru_0160 | mru_0160 | Adhesin-like protein | 1.02 | 6.70 | |
| mru_0963 | mru_0963 | Adhesin-like protein | 1.08 | 12.13 | |
| mru_1263 | mru_1263 | Adhesin-like protein | 1.15 | 9.15 | |
| mru_0331 | mru_0331 | Adhesin-like protein | 1.15 | 10.34 | |
| mru_0338 | mru_0338 | Adhesin-like protein | 1.17 | 8.55 | |
| mru_1124 | mru_1124 | Adhesin-like protein | 1.20 | 12.55 | |
| mru_0031 | mru_0031 | Adhesin-like protein | 1.27 | 11.29 | |
| mru_0687 | mru_0687 | Adhesin-like protein | 1.28 | 10.46 | |
| mru_0245 | mru_0245 | Adhesin-like protein | 1.32 | 8.78 | |
| mru_1417 | mru_1417 | Adhesin-like protein | 1.43 | 9.49 | |
| mru_1650 | mru_1650 | Adhesin-like protein | 1.44 | 4.24 | |
| mru_1465 | mru_1465 | Adhesin-like protein | 1.61 | 6.82 | |
| mru_1506 | mru_1506 | Adhesin-like protein | 1.61 | 7.76 | |
| mru_0417 | mru_0417 | Adhesin-like protein | 1.70 | 5.86 | |
| mru_0327 | mru_0327 | Adhesin-like protein | 1.73 | 10.86 | |
| mru_0019 | mru_0019 | Adhesin-like protein | 2.04 | 7.42 | |
| mru_0084 | mru_0084 | Adhesin-like protein | 2.07 | 6.71 | |
| mru_2049 | mru_2049 | Adhesin-like protein | 2.25 | 11.23 | |
| mru_2043 | mru_2043 | Adhesin-like protein | 2.27 | 8.58 | |
| mru_1726 | mru_1726 | Adhesin-like protein | 2.32 | 8.37 | |
| mru_2090 | mru_2090 | Adhesin-like protein | 2.51 | 13.88 | |
| mru_2147 | mru_2147 | Adhesin-like protein | 2.73 | 13.13 | |
| mru_0326 | mru_0326 | Adhesin-like protein | 5.04 | 12.58 | |
| mru_0015 | mru_0015 | Adhesin-like protein with cysteine protease domain | 1.49 | 9.07 | |
| mru_0020 | mru_0020 | Adhesin-like protein with cysteine protease domain | 2.78 | 7.86 | |
| Teichoic acid biosynthesis | mru_1079 | mru_1079 | CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase | 1.27 | 6.32 |
| Pseudomurein biosynthesis | mru_1118 | mru_1118 | Cell wall biosynthesis protein Mur ligase family | 1.07 | 9.37 |
| Cellular processes | |||||
| Oxidative stress response | mru_1507 | fprA1 | F420H2 oxidase FprA1 | 1.37 | 10.47 |
| mru_0131 | fprA2 | F420H2 oxidase FprA2 | 3.58 | 12.42 | |
| mru_1367 | rbr2 | Rubrerythrin Rbr2 | 1.27 | 13.19 | |
| Stress response | mru_0183 | mru_0183 | Protein disulfide-isomerase thioredoxin-related protein | − 1.19 | 7.79 |
| Central carbon metabolism | |||||
| Gluconeogenesis | mru_0628 | pgk2A | 2-Phosphoglycerate kinase Pgk2A | 1.85 | 7.69 |
| Other | mru_1685 | deoC | Deoxyribose-phosphate aldolase DeoC | 5.12 | 11.11 |
| Acetate | mru_1786 | mru_1786 | Transporter SSS family | − 1.18 | 8.66 |
| Energy metabolism | |||||
| Electron transfer | mru_0915 | mru_0915 | 4Fe–4S binding domain-containing protein | − 1.06 | 7.64 |
| mru_2036 | mru_2036 | 4Fe–4S binding domain-containing protein | 1.25 | 5.60 | |
| mru_1345 | mru_1345 | 4Fe–4S binding domain-containing protein | 1.30 | 7.63 | |
| Methanogenesis pathway | mru_0569 | mer | 5,10-methylenetetrahydro-methanopterin reductase Mer | − 1.36 | 12.71 |
| mru_0526 | hmd | Coenzyme F420-dependent N(5), N(10)-methenyltetrahydromethanopterin reductase Hmd | 1.41 | 10.96 | |
| mru_1850 | atwA2 | Methyl-coenzyme M reductase component A2 AtwA2 | 1.05 | 10.86 | |
| mru_1927 | mcrD | Methyl-coenzyme M reductase D subunit McrD | − 1.43 | 11.33 | |
| mru_0441 | mtrA2 | Tetrahydromethanopterin S-methyltransferase subunit A MtrA2 | − 2.14 | 11.99 | |
| mru_1918 | mtrF | Tetrahydromethanopterin S-methyltransferase subunit F MtrF | − 1.24 | 9.71 | |
| Electron transfer | mru_0184 | dsbD | Cytochrome C-type biogenesis protein DsbD | − 1.16 | 6.17 |
| mru_0830 | mru_0830 | Ferredoxin | 2.56 | 9.31 | |
| H2 metabolism | mru_1410 | ehaC | Energy-converting hydrogenase A subunit C EhaC | − 1.63 | 6.30 |
| mru_1408 | ehaE | Energy-converting hydrogenase A subunit E EhaE | − 1.74 | 7.34 | |
| mru_1632 | hypB | Hydrogenase accessory protein HypB | 2.25 | 7.90 | |
| mru_1633 | hypA | Hydrogenase nickel insertion protein HypA | 2.19 | 7.47 | |
| Formate metabolism | mru_0332 | fdhC | Formate/nitrite transporter FdhC | − 1.11 | 11.98 |
| Alcohol metabolism | mru_1445 | adh3 | NADP-dependent alcohol dehydrogenase Adh3 | 6.42 | 7.81 |
| mru_1444 | npdG2 | NADPH-dependent F420 reductase NpdG2 | 3.84 | 5.32 | |
| Mobile elements | |||||
| Prophage | mru_0269 | mru_0269 | ATPase involved in DNA replication control MCM family | 2.51 | 4.60 |
| mru_0323 | mru_0323 | dnd system-associated protein 2 | 1.11 | 6.63 | |
| mru_0280 | mru_0280 | ParB-like nuclease domain-containing protein | 2.52 | 1.87 | |
| mru_0256 | mru_0256 | Phage integrase | 1.69 | 6.95 | |
| mru_0287 | mru_0287 | Phage portal protein | 2.73 | 1.86 | |
| mru_0315 | mru_0315 | Phage tail tape measure protein | 2.47 | 3.39 | |
| mru_0270 | mru_0270 | Phage-related protein | 1.91 | 4.54 | |
| mru_0288 | mru_0288 | Phage-related protein | 2.21 | 2.32 | |
| mru_0058 | mru_0058 | Phage-related protein | 2.53 | − 0.04 | |
| mru_0282 | mru_0282 | Phage-related protein | 2.64 | 1.93 | |
| mru_0316 | mru_0316 | Phage-related protein | 2.66 | 3.40 | |
| mru_0317 | mru_0317 | Phage-related protein | 2.89 | 3.42 | |
| mru_0311 | mru_0311 | Phage-related protein | 3.14 | 2.55 | |
| mru_0310 | mru_0310 | Phage-related protein | 3.18 | 1.56 | |
| mru_0284 | mru_0284 | Phage-related protein | 3.35 | 1.93 | |
| mru_0307 | mru_0307 | Phage-related protein | 3.38 | 2.86 | |
| mru_0313 | mru_0313 | Phage-related protein | 3.40 | 2.83 | |
| mru_0308 | mru_0308 | Phage-related protein | 3.48 | 3.46 | |
| mru_0324 | mru_0324 | Type II restriction enzyme, methylase subunit | 1.88 | 5.99 | |
| CRISPR-associated genes | mru_0798 | mru_0798 | CRISPR-associated protein Cas1-1 | 1.93 | 4.09 |
| mru_1181 | mru_1181 | CRISPR-associated RAMP protein Csm3 family | 1.03 | 7.23 | |
| Nitrogen metabolism | |||||
| Other | mru_2121 | hcp | Hydroxylamine reductase Hcp | − 1.46 | 12.26 |
| Nucleic acid metabolism | |||||
| Helicase | mru_0981 | mru_0981 | Rad3-related DNA helicase | 1.09 | 7.97 |
| Recombination and repair | mru_2097 | recJ1 | ssDNA exonuclease RecJ1 | 1.39 | 11.06 |
| mru_1383 | mru_1383 | Staphylococcal nuclease domain-containing protein | − 1.30 | 7.06 | |
| Protein fate | |||||
| Protein folding | mru_1511 | mru_1511 | Nascent polypeptide-associated complex protein | − 1.00 | 6.61 |
| Protein secretion | mru_1581 | mru_1581 | Signal peptidase I | − 1.21 | 7.34 |
| Protein synthesis | |||||
| RNA processing | mru_0589 | mru_0589 | NMD3 family protein | 1.50 | 7.52 |
| Translation factors | mru_0728 | mru_0728 | Peptide chain release factor aRF1 | 1.46 | 7.74 |
| Ribosomal proteins | mru_0865 | rpl5p | Ribosomal protein L5P Rpl5p | 1.03 | 8.24 |
| mru_0868 | rpl6p | Ribosomal protein L6P Rpl6p | 1.05 | 7.92 | |
| mru_2098 | mru_2098 | Ribosomal protein S15P Rps15p | 1.19 | 9.21 | |
| Other | mru_0519 | mru_0519 | RNA-binding protein | − 1.68 | 8.08 |
| mru_1978 | mru_1978 | RNA-metabolising metallo-beta-lactamase | 1.58 | 8.74 | |
| RNA processing | mru_1846 | dusA2 | tRNA-dihydrouridine synthase DusA2 | 1.06 | 6.58 |
| Purines and pyrimidines | |||||
| Interconversion | mru_2104 | surE1 | 5′-Nucleotidase SurE1 | 1.02 | 7.02 |
| mru_0241 | nrdD | Anaerobic ribonucleoside-triphosphate reductase NrdD | 1.47 | 11.08 | |
| Regulation | |||||
| Protein interaction | mru_1186 | mru_1186 | TPR repeat-containing protein | − 1.05 | 8.81 |
| Transcriptional regulator | mru_2122 | mru_2122 | Transcriptional regulator | − 1.62 | 8.68 |
| mru_1447 | mru_1447 | Transcriptional regulator | − 1.55 | 8.56 | |
| mru_1446 | mru_1446 | Transcriptional regulator ArsR family | − 1.21 | 7.78 | |
| mru_0442 | mru_0442 | Transcriptional regulator MarR family | − 1.68 | 4.74 | |
| Secondary metabolites | |||||
| Other | mru_0514 | mru_0514 | 4′-Phosphopantetheinyl transferase family protein | 1.26 | 6.32 |
| mru_0069 | mru_0069 | MatE efflux family protein | 1.20 | 7.17 | |
| mru_0352 | mru_0352 | MatE efflux family protein | 1.64 | 6.73 | |
| NRPS | mru_0351 | mru_0351 | Non-ribosomal peptide synthetase | 1.06 | 10.17 |
| Transcription | |||||
| RNA polymerase | mru_0161 | rpoF | DNA-directed RNA polymerase subunit F RpoF | 1.05 | 9.66 |
| Transporters | |||||
| Amino acids | mru_1775 | mru_1775 | Amino acid ABC transporter ATP-binding protein | 1.03 | 5.46 |
| mru_1776 | mru_1776 | Amino acid ABC transporter permease protein | 1.25 | 4.94 | |
| Cations | mru_1861 | mru_1861 | Heavy metal translocating P-type ATPase | − 6.61 | 10.24 |
| mru_1706 | nikD2 | Nickel ABC transporter ATP-binding protein NikD2 | 1.15 | 6.54 | |
| mru_1617 | nikB1 | Nickel ABC transporter permease protein NikB1 | 1.10 | 7.35 | |
| mru_1709 | nikB2 | Nickel ABC transporter permease protein NikB2 | 1.43 | 7.34 | |
| mru_1708 | nikC2 | Nickel ABC transporter permease protein NikC2 | 1.31 | 7.03 | |
| mru_1710 | nikA2 | Nickel ABC transporter substrate-binding protein NikA2 | 1.14 | 11.86 | |
| Other | mru_0253 | mru_0253 | ABC transporter ATP-binding protein | 1.97 | 7.23 |
| mru_0252 | mru_0252 | ABC transporter permease protein | 1.71 | 7.40 | |
| mru_0251 | mru_0251 | ABC transporter substrate-binding protein | 2.06 | 9.13 | |
| mru_0329 | mru_0329 | MotA/TolQ/ExbB proton channel family protein | 1.56 | 6.00 | |
| Vitamins and cofactors | |||||
| Biotin | mru_0527 | bioB2 | Biotin synthase BioB2 | 1.24 | 7.09 |
| Cobalamin | mru_0539 | cbiM1 | Cobalamin biosynthesis protein CbiM1 | 1.21 | 9.82 |
| mru_0540 | cbiN1 | Cobalt transport protein CbiN1 | 1.18 | 8.30 | |
| mru_0360 | cbiA1 | Cobyrinic acid a,c-diamide synthase CbiA1 | − 1.60 | 8.09 | |
| mru_1852 | cysG | Siroheme synthase CysG | 1.20 | 7.47 | |
| Coenzyme B | mru_0385 | aksA | Homocitrate synthase AksA | − 1.15 | 10.22 |
| Metal-binding pterin | mru_0200 | modB | Molybdate ABC transporter permease protein ModB | 2.04 | 9.37 |
| mru_0201 | modA | Molybdate ABC transporter substrate-binding protein ModA | 2.83 | 10.54 | |
| Thiamine | mru_0247 | thiC1 | Thiamine biosynthesis protein ThiC1 | − 1.18 | 9.24 |
| mru_0532 | mru_0532 | ThiF family protein | 1.38 | 4.67 | |
| Others | mru_1769 | nifB | Nitrogenase cofactor biosynthesis protein NifB | 2.58 | 8.89 |
| Unknown function | |||||
| Enzyme | mru_0455 | mru_0455 | Acetyltransferase | − 1.16 | 9.80 |
| mru_1758 | mru_1758 | Acetyltransferase | − 1.10 | 6.05 | |
| mru_2170 | mru_2170 | Acetyltransferase | 1.32 | 6.12 | |
| mru_0574 | mru_0574 | Acetyltransferase GNAT family | − 1.92 | 1.81 | |
| mru_1707 | mru_1707 | Acetyltransferase GNAT family | 1.48 | 5.54 | |
| mru_0560 | mru_0560 | ATPase | 1.11 | 8.14 | |
| mru_1613 | mru_1613 | SAM-dependent methyltransferase | 1.58 | 4.18 | |
| Other | mru_0231 | mru_0231 | CAAX amino terminal protease family protein | − 1.09 | 8.53 |
| mru_1993 | mru_1993 | CBS domain-containing protein | − 1.65 | 10.72 | |
| mru_1994 | mru_1994 | CBS domain-containing protein | − 1.31 | 11.57 | |
| mru_0474 | mru_0474 | HD domain-containing protein | 1.33 | 7.47 | |
| mru_1034 | mru_1034 | HEAT repeat-containing protein | 2.35 | 8.75 | |
| mru_2109 | mru_2109 | Methanogenesis marker protein 12 | − 1.01 | 7.90 | |
| mru_0562 | mru_0562 | PP-loop family protein | 1.59 | 7.50 | |
| mru_1678 | mru_1678 | Redox-active disulfide protein | 1.51 | 7.12 | |
| mru_0561 | mru_0561 | Von Willebrand factor type A domain-containing protein | 1.33 | 8.52 | |
| mru_1510 | mru_1510 | YhgE/Pip-like protein | − 1.31 | 8.45 | |
| mru_0627 | mru_0627 | ZPR1 zinc-finger domain-containing protein | 2.04 | 6.70 | |
C12-treated cultures were compared to DSMO-exposed control cultures (significant change with log2fold changes < 1 and > 1 and a false discovery rate < 0.05). The list does not include the 71 regulated hypothetical proteins. The M. ruminantium (mru) open reading frame (ORF) codes are adopted from the Kyoto Encyclopedia of Genes and Genomes
The subcellular localization of the encoded protein could be identified for 75% of the regulated genes. Predominantly, genes associated with the cell envelope were affected, namely trans-membrane proteins or membrane-associated proteins. Enrichment analysis showed that, with C12 exposure, mainly adhesion-like proteins (category ‘cell surface’) and phage-related proteins (‘mobile elements’) were significantly enriched in the regulated genes data set (Table 1). This supports earlier suggestions that MCFA primarily target the cell envelope and processes that occur at the cell membrane [12]. For example, upon exposure to C12 in the present study, the mRNA abundance of 26 adhesion-like proteins (ALPs) (part of the cell envelope [13]), i.e. of 25% of all ALPs of M. ruminantium, and of two proteins involved in biosynthesis of teichoic acid and pseudomurein which are cell-wall related [14], were up-regulated compared to the DMSO control group (Table 2).
Two subunits of the membrane-bound energy-converting hydrogenase (Eha), which is involved in hydrogenotrophic methanogenesis [13, 15], were down-regulated by log21.6- and 1.7-fold in cultures exposed to C12, whereas two cytoplasmic hydrogenases (Frh, Mvh) were not. A gene encoding ferredoxin, a trans-membrane iron-sulfur protein involved in electron transfer from hydrogen, was up-regulated (log 2.6-fold upon C12 exposure). Expression of 3 genes encoding trans-membrane 4Fe-4S binding domain-containing proteins was affected by C12 exposure. Two subunits of the methyl-H4MPT:coenzyme M methyltransferase (Mtr), which is membrane-bound and plays a crucial role in the methanogenesis pathway [15, 16], were down-regulated by log2 2.1- and 1.2-fold upon C12 exposure. In total 13 genes encoding mainly transporters of amino acids and cations displayed differences in transcript abundance after C12 exposure (Table 2). For example, several genes encoding subunits of cations transporters, like the nickel ABC transporter permease proteins or nickel ABC transporter ATP-binding proteins, NikA2, NikB1, NikB2, NikC2 and NikD2, were differentially regulated. These cation transporters belong to a large family of ABC transporters (peptide/nickel transporter family) in ABC-type nickel transporter system, which is composed of a periplasmic binding protein (NikA), two integral membrane proteins (NikB and NikC) and two ABC proteins (NikD and NikE) [17]. One P-type ATPase, which are membrane-bound efflux pumps involved in metal homeostasis of microorganisms [18], was down-regulated. In prokaryotes, ABC transporters and P-type ATPases have important functions in maintaining appropriate concentrations of transition metals such as Ni, Co, Fe, Cu, and Zn, which are essential components of many prokaryotic enzymes [18]. Two transmembrane cobalt transport proteins (mru_0540; mru_0539), and two membrane-associated proteins involved in molybdate transport (mru_0200, mru_0201) [19], were up-regulated.
In addition, genes encoding intracellular proteins were affected by C12 exposure. These data support earlier observations that exposure to C12 causes leakage of intracellular K+ ions in M. ruminantium [6, 7], thus damages the cell envelope. Amongst the regulated genes, mostly genes encoding proteins involved in DNA repair, and genes controlling transcription/translation and redox homeostasis were affected. For example, thioredoxins and rubrerythrins showed an altered expression; they are considered to form a system protecting Archaea against oxidative stress [20, 21]. Thioredoxin-like proteins exhibit biochemical activities similar to thioredoxin and help methanogens maintain redox homeostasis [7]. Genes which were up-regulated by C12 included genes encoding proteins that are involved in nucleic acid metabolism and repair and in translation include a helicase (mru_0981), an exonuclease (mru_2097, recJ1), an anaerobic ribonucleosid-triphosphate reductase nrdD (mru_0241), a nucleotidase (mru_2104; SurE1), and a RNA-metabolizing metallo-beta-lactamase (mru_1978). Several genes involved in translation or post-translational modification were down-regulated, e.g. a staphylococcal nuclease domain-containing protein (mru_1383), a nascent polypeptide-associated complex protein (mru_1511), an RNA-binding protein (mru_0519) and a signal peptidase (mru_1581).
Conclusion
The transcriptional response of M. ruminantium to the fatty acid C12 does not involve repression of specific pathway such as the methanogenesis pathway. Instead, it implies that C12 provokes broad transcriptional changes, and targets primarily cell surface associated adhesion-like proteins, phage-related proteins, and transmembrane proteins. How this response affects methanogens remains unclear. Future studies may investigate how different dosages of and prolonged exposure to C12 affect gene and protein expression and survival of M. ruminantium.
Limitations
One limitation of our study is the low number of replicates per group. In addition, only one dosage of C12 was tested and samples for RNA sequencing were collected only at one time point; this precludes generalization to situations where C12 affects M. ruminantium stronger or weaker.
Additional files
Additional file 1: Table S1. M. ruminantium M1 genes with significantly changed expression of genes in the DMSO control as compared to the blank group (log2-fold change < 1 and > 1, false discovery rate < 0.05). The list does not include the 59 regulated hypothetical proteins. The M. ruminantium (mru) open reading frame (ORF) codes are adopted from the Kyoto Encyclopedia of Genes and Genomes.
Additional file 2: Table S2. M. ruminantium M1 genes with significantly changed expression of genes in the cultures exposed to C12 + DMSO as compared to the blank group (log2-fold change < 1 and > 1, false discovery rate < 0.05). The list does not include the 15 regulated hypothetical proteins. The M. ruminantium (mru) open reading frame (ORF) codes are adopted from the Kyoto Encyclopedia of Genes and Genomes.
Additional file 3: Figure S1. Venn diagram indicates the number of differentially expressed genes between the experimental groups and the common overlapping differentially expressed genes. TC: treatment (C12 + DMSO) vs. control (DMSO); TB: treatment (C12 + DMSO) vs. untreated blank; CB: control (DMSO) vs. untreated blank. It should be kept in mind that it is not possible to distinguish between the DMSO and the C12 effect in the dataset comparing the treatment and the blank samples, and that the C12 effect is much better studied in the TC comparison (C12 + DMSO vs DMSO). The DMSO effect can be partial quenched by the C12 effect, so genes regulated in CB and TC are not necessarily regulated in the TB. The 26 common genes differentially expressed in M. ruminantium exposed to DMSO or DMSO + C12 compared to the untreated blank control are outlined in the tables on the right side. The 35 overlapping differentially expressed genes of the TC and CB comparisons are outlined in the table on the left side. The diagram was generated using the online tool at bioinformatics.psb.ugent.be/webtools/Venn/.
Authors’ contributions
XZ participated in designing the study, performed the data collection, and drafted the manuscript. MJAS performed the data analysis and contributed to data interpretation. SN participated in designing the study, data collection and data interpretation and revised the manuscript. AS participated in data collection and critically revised the manuscript. MK participated in designing the study and critically revised the manuscript. AB participated in designing the study, performed the sequencing experiment, wrote the methods section of the manuscript, contributed to interpretation of the data and revised the manuscript. JOZ designed the study and wrote introduction, results and discussion of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The raw data can be accessed in the NCBI Sequence Read Archive (SRA) under the series record GSE81199 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81199.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This study was supported by the China Scholarship Council and the ETH Zurich Scholarship for Doctoral Students.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MCFA
medium-chain fatty acids
- C12
lauric acid
- DMSO
dimethyl sulfoxide
- CH4
methane
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3242-8) contains supplementary material, which is available to authorized users.
Anna Bratus-Neuenschwander and Johanna O. Zeitz contributed equally to this work
Contributor Information
Xuan Zhou, Email: evezxo@gmail.com.
Marc J. A. Stevens, Email: marc.stevens@uzh.ch
Stefan Neuenschwander, Email: stefan.neuenschwander@usys.ethz.ch.
Angela Schwarm, Email: angela.schwarm@usys.ethz.ch.
Michael Kreuzer, Email: michael.kreuzer@inw.agrl.ethz.ch.
Anna Bratus-Neuenschwander, Email: abratus@usys.ethz.ch.
Johanna O. Zeitz, Email: jzeitz@gmx.de
References
- 1.UNFCC 2016 (United Nations Framework Convention on Climate Change). Greenhouse Gas Data, unfccc.int/ghg_data/ghg:data:unfccc/items/4146.php. Accessed 5 Sept 2016.
- 2.Jordan E, Lovett DK, Hawkins M, Callan JJ, O’Mara FP. The effect of varying levels of coconut oil on intake, digestibility and methane output from continental cross beef heifers. Anim Sci. 2006;82:859–865. doi: 10.1017/ASC2006107. [DOI] [Google Scholar]
- 3.Machmüller A, Kreuzer M. Methane suppression by coconut oil and associated effects on nutrient and energy balance in sheep. Can J Anim Sci. 1999;79:65–72. doi: 10.4141/A98-079. [DOI] [Google Scholar]
- 4.Machmüller A, Soliva CR, Kreuzer M. Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion. Br J Nutr. 2003;90:529–540. doi: 10.1079/BJN2003932. [DOI] [PubMed] [Google Scholar]
- 5.Dohme F, Machmüller A, Wasserfallen A, Kreuzer M. Ruminal methanogenesis as influenced by individual fatty acids supplemented to complete ruminant diets. Lett Appl Microbiol. 2001;32:47–51. doi: 10.1046/j.1472-765x.2001.00863.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Meile L, Kreuzer M, Zeitz JO. The effect of saturated fatty acids on methanogenesis and cell viability of Methanobrevibacter ruminantium. Archaea. 2013;2013:106916. doi: 10.1155/2013/106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Zeitz JO, Meile L, Kreuzer M, Schwarm A. Influence of pH and the degree of protonation on the inhibitory effect of fatty acids in the ruminal methanogen Methanobrevibacter ruminantium strain M1. J Appl Microbiol. 2015;119:1482–1493. doi: 10.1111/jam.12955. [DOI] [PubMed] [Google Scholar]
- 8.Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/S0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Tanner SA, Chassard C, Rigozzi E, Lacroix C, Stevens MJA. Bifidobacterium thermophilum RBL67 impacts on growth and virulence gene expression of Salmonella enterica subsp. enterica serovar Typhimurium. BMC Microbiol. 2016;16:46–61. doi: 10.1186/s12866-016-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal AZ, Matson EG, Eldar A, Leadbetter JR. RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. ISME J. 2011;5:1133–1142. doi: 10.1038/ismej.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas BJ, Chin M, Nusbaum C, Birren BW, Livny J. How deep is deep enough for RNA-Seq profiling of bacterial transcriptomes? BMC Genom. 2012;13:734–744. doi: 10.1186/1471-2164-13-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 13.Leahy SC, Kelly WJ, Altermann E, Ronimus RS, Yeoman CJ, Pacheco DM, et al. The genome sequence of the ruminal methanogen Methanobrevibacter ruminantium reveals new possibilities for controlling ruminant methane emissions. PLoS ONE. 2010;5:e8926. doi: 10.1371/journal.pone.0008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beld J, Sonnenschein EC, Vickery CR, Noelb JP, Burkart MD. The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life. Nat Prod Rep. 2014;31:61–108. doi: 10.1039/C3NP70054B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation, Nature Rev. Microbiol. 2008;8:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- 16.Kaster AK, Goenrich M, Seedorf H, Liesegang H, Wollherr A, Gottschalk G, Thauer RK. More than 200 genes required for methane formation from H2 and CO2 and energy conservation are present in Methanothermobacter marburgensis and Methanothermobacter thermautotrophicus. Archaea. 2011;2011:973848. doi: 10.1155/2011/973848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodionov DA, Hebbeln P, Gelfand MS, Eitinger T. Comparative and functional genomic analysis of prokaryotic nickel and cobalt uptake transporters: evidence for a novel group of ATP-binding cassette transporters. J Bacteriol. 2006;188:317–327. doi: 10.1128/JB.188.1.317-327.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewinson O, Lee AT, Rees DC. A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci. 2009;106:4677–4682. doi: 10.1073/pnas.0900666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunden AM, Shanmugam KT. Molybdate transport and regulation in bacteria. Arch Microbiol. 1997;168:345–354. doi: 10.1007/s002030050508. [DOI] [PubMed] [Google Scholar]
- 20.Pedone E, Bartolucci S, Fiorentino G. Sensing and adapting to environment stress: the archaeal tactic. Front Biosci. 2004;9:2909–2926. doi: 10.2741/1447. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Kosaka T, Watanabe K. Comparative transcriptome analysis of responses of Methanothermobacter thermautotrophicus to different environmental stimuli. Environ Microbiol. 2008;10:893–905. doi: 10.1111/j.1462-2920.2007.01508.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. M. ruminantium M1 genes with significantly changed expression of genes in the DMSO control as compared to the blank group (log2-fold change < 1 and > 1, false discovery rate < 0.05). The list does not include the 59 regulated hypothetical proteins. The M. ruminantium (mru) open reading frame (ORF) codes are adopted from the Kyoto Encyclopedia of Genes and Genomes.
Additional file 2: Table S2. M. ruminantium M1 genes with significantly changed expression of genes in the cultures exposed to C12 + DMSO as compared to the blank group (log2-fold change < 1 and > 1, false discovery rate < 0.05). The list does not include the 15 regulated hypothetical proteins. The M. ruminantium (mru) open reading frame (ORF) codes are adopted from the Kyoto Encyclopedia of Genes and Genomes.
Additional file 3: Figure S1. Venn diagram indicates the number of differentially expressed genes between the experimental groups and the common overlapping differentially expressed genes. TC: treatment (C12 + DMSO) vs. control (DMSO); TB: treatment (C12 + DMSO) vs. untreated blank; CB: control (DMSO) vs. untreated blank. It should be kept in mind that it is not possible to distinguish between the DMSO and the C12 effect in the dataset comparing the treatment and the blank samples, and that the C12 effect is much better studied in the TC comparison (C12 + DMSO vs DMSO). The DMSO effect can be partial quenched by the C12 effect, so genes regulated in CB and TC are not necessarily regulated in the TB. The 26 common genes differentially expressed in M. ruminantium exposed to DMSO or DMSO + C12 compared to the untreated blank control are outlined in the tables on the right side. The 35 overlapping differentially expressed genes of the TC and CB comparisons are outlined in the table on the left side. The diagram was generated using the online tool at bioinformatics.psb.ugent.be/webtools/Venn/.
Data Availability Statement
The raw data can be accessed in the NCBI Sequence Read Archive (SRA) under the series record GSE81199 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81199.


