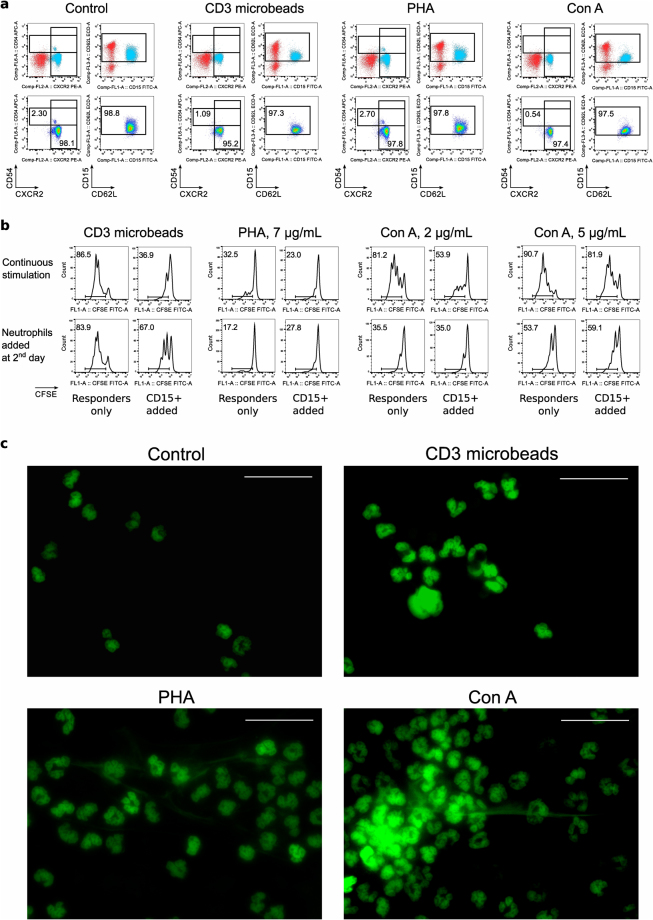
Figure 6.
CD15+ neutrophils may be directly stimulated by lectins. Healthy donor CD15+ cells were incubated for 2 hours at 37 °C in cell culture media in presence of anti-CD3 microbeads (3.5 beads per cell), PHA (7 µg/mL) or Con A (5 µg/mL), then evaluated with flow cytometry for live/dead, CD15 and activation markers: CXCR2, CD54 and CD62L. On top: location of CD15+SSChi population of neutrophils is shown by backgating (neutrophils are blue). On the second row: neutrophils preserve their naïve phenotype. One representative example of cells, isolated the same day as blood drawn, is showed. At least 3 samples from each group, either first day blood or second day blood samples, were evaluated in 5 independent experiments. (b) Flow plots showing CFSE-proliferation of CD4+ responder cells in suppression assays. Healthy donor CD15+ cells were used in suppression assay with CFSE-labeled healthy donor responders PBMC in 1/1 ratios. On top: neutrophils were incubated with PBMC in presence of anti-CD3 microbeads or lectins, and responders showed signs of suppression. On the bottom: same responders PBMC were pre-activated with the same mitogens for overnight, then washed and mixed 1:1 with neutrophils. In absence of lectins, neutrophils had no effect (or even some stimulatory effects) on T cell divisions, but still able to disrupt T cell activation by anti-CD3 microbeads. In total, 3 experiments with 4 healthy donors’ neutrophils and 8 healthy donors’ PBMC responders were performed with similar results. Histograms and dot plots consist of 12,695 ± 2891 (Mean ± SEM) events. (c) Microscopic evaluation of CD15+ cells after 1 hour incubation with anti-CD3 microbeads, PHA or Con A. Sytox green stained cells showed an absence of aggregation and NET production in control condition, but signs of aggregation and NET production with both lectins. Anti-CD3 microbeads, as it was shown in light microscopy, were aggregated with neutrophils and may be seen as round unstained shapes. One experiment out of two is shown. More details are shown in Suppl. Figures 10–15.