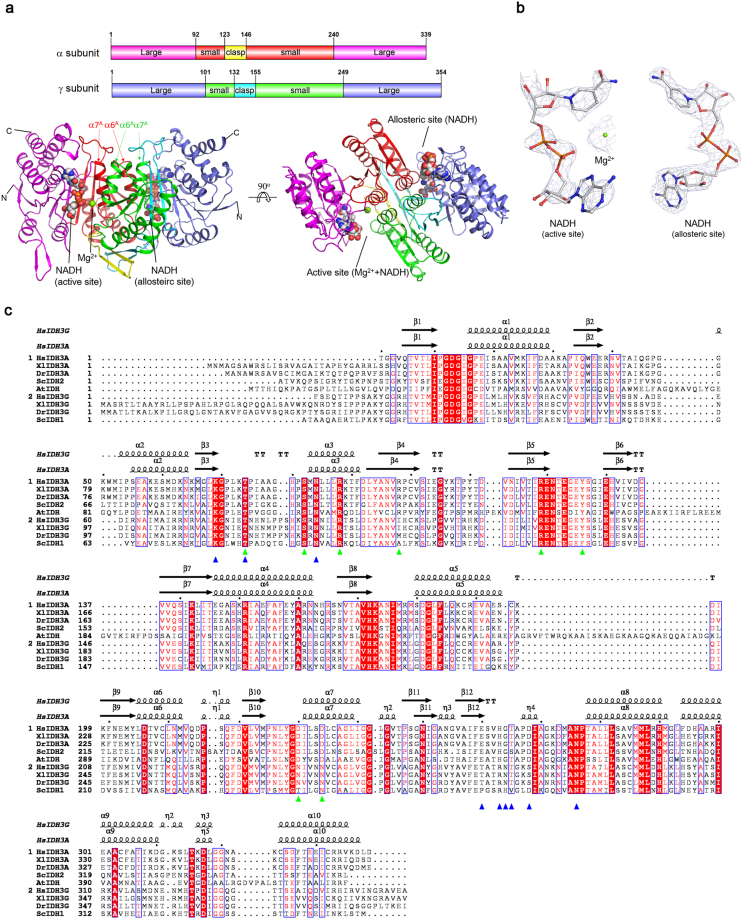
Figure 3.
Structure of the αMg+NADHγNADH heterodimer of human NAD-IDH. (a) Overall structure of the αMg+NADHγNADH heterodimer in two different views. Left: view in perpendicular to the pseudo 2-fold axis of the αγ heterodimer. Right: view along the pseudo 2-fold axis of the αγ heterodimer. The color-coding schemes of individual domains of the α and γ subunits are shown above. The bound NADHs are shown in space-filling models, and the bound Mg2+ ion as a green sphere. (b) Representative simulated annealing composite omit maps (contoured at 1.0σ level) of the bound NADHs in the αMg+NADHγNADH structure. Left: Mg2+ and NADH at the active site. Right: NADH at the allosteric site. The NADH is shown in ball-and-stick model and the Mg2+ with a green sphere, respectively. (c) Structure-based sequence alignment of the α and γ subunits of human NAD-IDH with several representative NAD-IDHs. Homo sapien NAD-IDH: HsIDH3; S. cerevesiae NAD-IDH: ScIDH; Xenopus laevis NAD-IDH: XlIDH3; Danio rerio NAD-IDH: DrIDH3; Acidithiobacillus thiooxidans NAD-IDH: AtIDH. The secondary structures of the α and γ subunits in the αMg+NADHγNADH structure are placed on the top of the alignment. Invariant residues are highlighted by shaded red boxes and conserved residues by open blue boxes. The residues corresponding to those composing the ICT-binding site and the NAD-binding site in the αγ heterodimer of human NAD-IDH are highlighted with green and blue triangles, respectively.