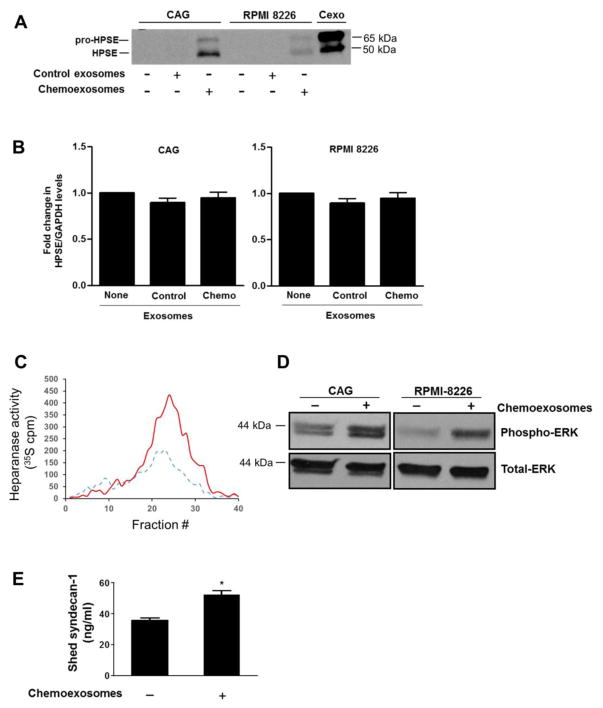
Fig. 3.
Chemoexosomes transfer heparanase enzyme activity to recipient cells. (A), Wild-type CAG or RPMI 8226 myeloma cells that express very low levels of endogenous heparanase were incubated for 16 hours with exosomes secreted by cells that were untreated (control exosomes) or treated with 250 nM bortezomib (chemoexosomes). Cells were washed to remove exosomes remaining in the medium then lysed and analyzed by western blotting for heparanase. Purified chemoexosomes (Cexo) were loaded in the lane on the far right of the gel. (B) Heparanase transcript levels in wild-type CAG and RPMI 8226 cells were assessed by quantitative PCR in the absence of exosomes or after 16 h exposure to control exosomes or chemoexosomes. (C) Cell lysates were prepared from wild-type CAG cells that had been incubated for 16 h with exosomes secreted by CAG-HPSE cells treated without (control exosomes, dashed line) or with bortezomib (chemoexosomes, solid line). The lysates were then added to a well containing 35S-labeled ECM and the liberated heparan sulfate degradation fragments were separated on a Sepharose 6B column. (D) Wild-type CAG or RPMI 8226 cells growing in the absence of presence of chemoexosomes were extracted and probed for levels of phospho-ERK or total ERK. (E) CAG wild-type cells were grown in the absence or presence of chemoexosomes and the amount of syndecan-1 shed into the culture medium after 16 hours was measured by ELISA. Results are mean ± SD from three separate experiments, *p<0.01 compared to no exosome addition.